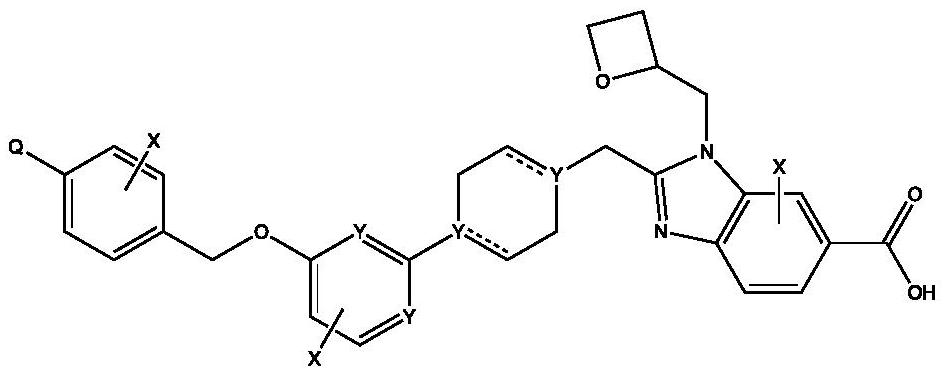
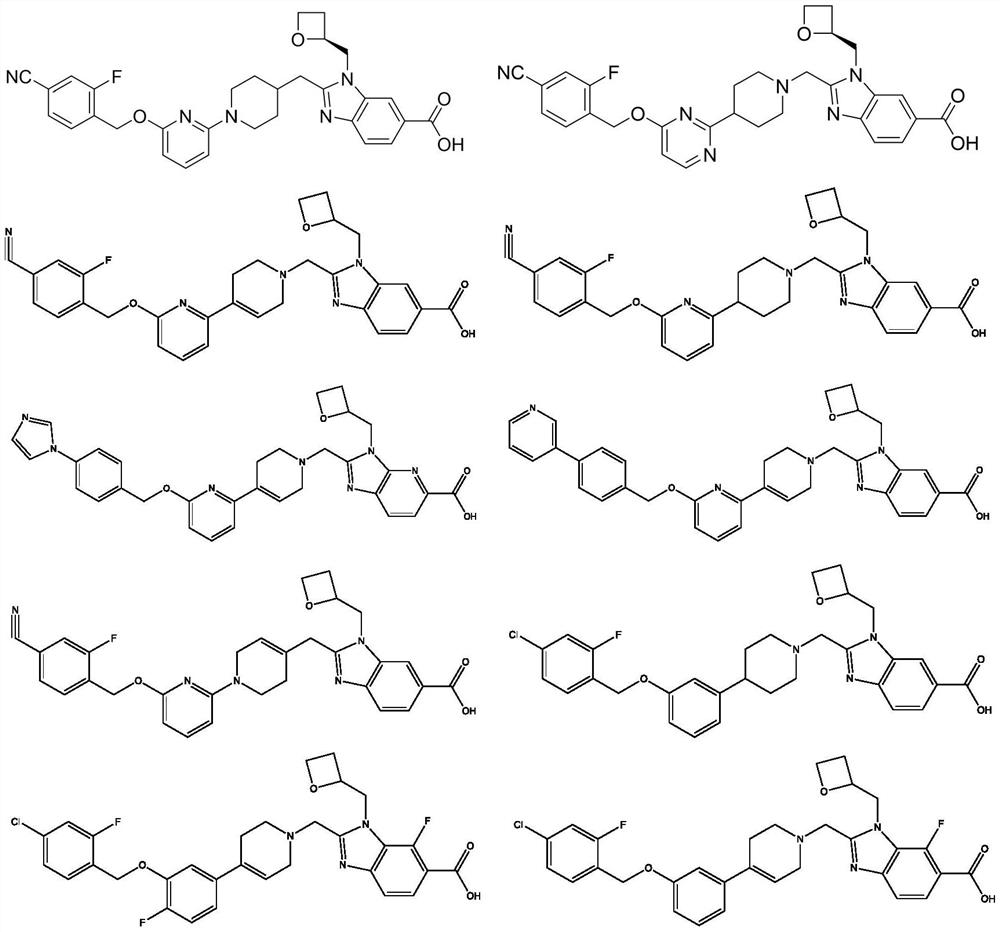
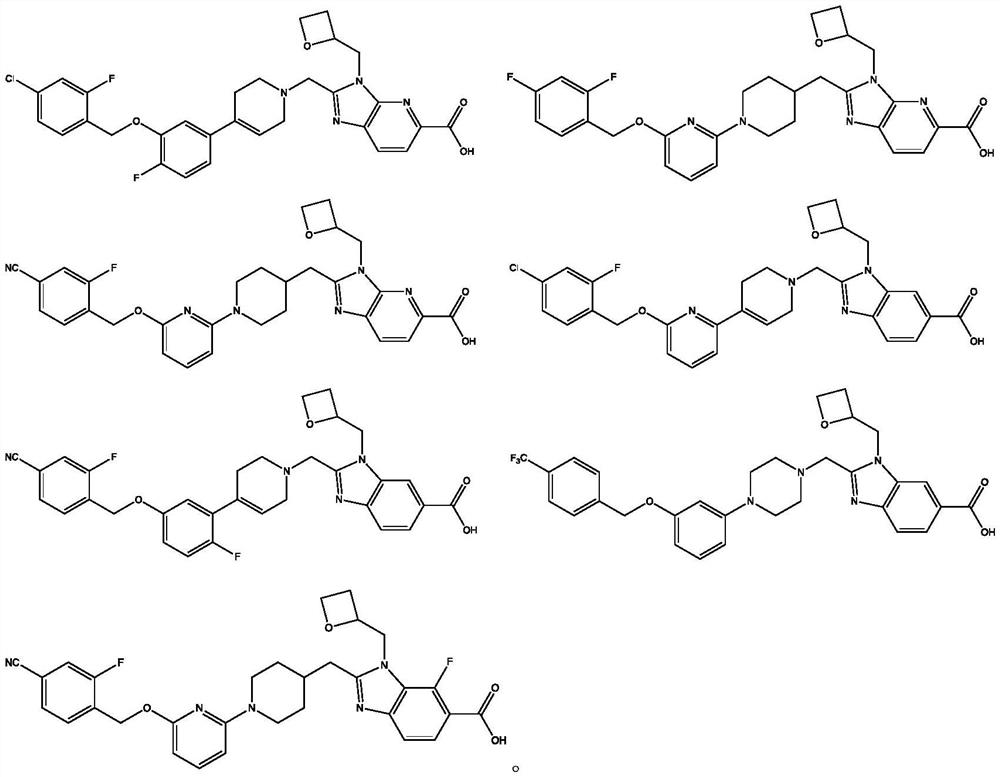
GLP-1 small molecule with cardiovascular benefits
A GLP-1, small molecule technology, applied in organic active ingredients, medical formulations containing active ingredients, organic chemistry, etc., can solve the non-inferiority placebo group, the cardiovascular benefit does not show a significant difference , lack of cardiovascular benefits, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0117] The present invention will be further described in detail below in conjunction with specific examples. The following examples are used to understand the method and core idea of the present invention. For those skilled in the art, any possible changes or substitutions are within the protection scope of the present invention without departing from the concept of the present invention. Unless otherwise specified, the raw materials and reagents used in the present invention are chemically pure or above.
[0118] 1. Preparation of Exemplary Compounds
[0119] Compound A
[0120] (1) Preparation of intermediates
[0121]
[0122] Diisopropylamine (1.84mL, 13.12mmol) was dissolved in tetrahydrofuran (12mL) and cooled to -26°C, n-BuLi (2.5M, 5.2mL, 13mmol) was added dropwise within fifteen minutes. After cooling down to -30°C, compound 1a (3.12g, 12.82mmol) was dissolved in tetrahydrofuran (10mL) and added dropwise. After half an hour of reaction, compound 1 (1.88g, 12....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com