Process for producing bromine by electrolytically acidizing sodium bromide
A process method and sodium bromide technology are applied in the directions of electrolysis components, electrolysis processes, electrodes, etc., which can solve the problems of large consumption of chlorine gas, and achieve the effects of reducing potential safety hazards, improving economic benefits, and meeting environmental protection requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
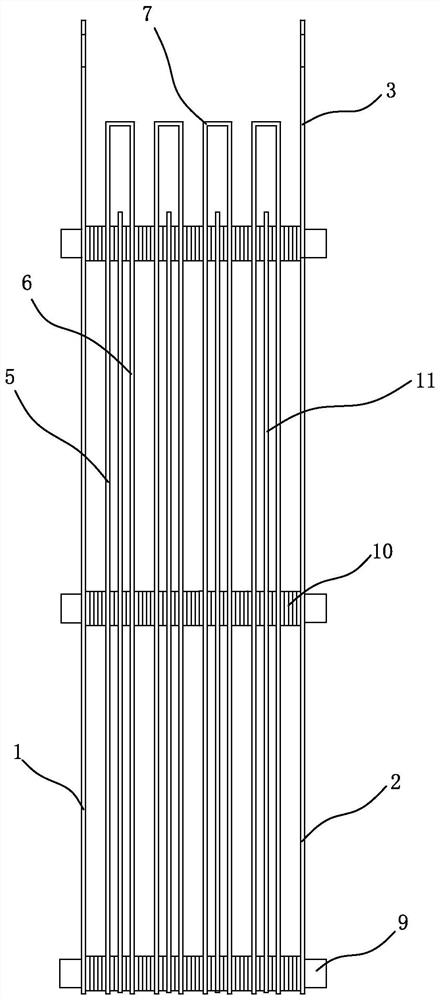
[0051] Such as Figure 2-6 As shown, an electrolytic cell includes a closed outer casing, an electrolytic device is arranged inside the outer casing, an electrolyte inlet is arranged at the bottom of the electrolytic cell, and a liquid overflow is arranged at the upper part of the electrolytic cell mouth, the top of the electrolytic cell is provided with a gas outlet.
[0052] The electrolysis device includes a cathode end plate 1 and an anode end plate 2 arranged in parallel, and the terminal plate 3 on the upper part of the cathode end plate 1 and the anode end plate 2 is respectively connected to the negative and positive poles of an external power supply. In this embodiment, the Four groups of unit cells 4 are arranged between the cathode end plate 1 and the anode end plate 2, and the unit cells 4 include an anode plate 5, a cathode plate 6, and a zigzag-shaped plate connected to the anode plate 5 and the cathode plate 6 respectively. The connection plate 7, the anode pla...
Embodiment 2
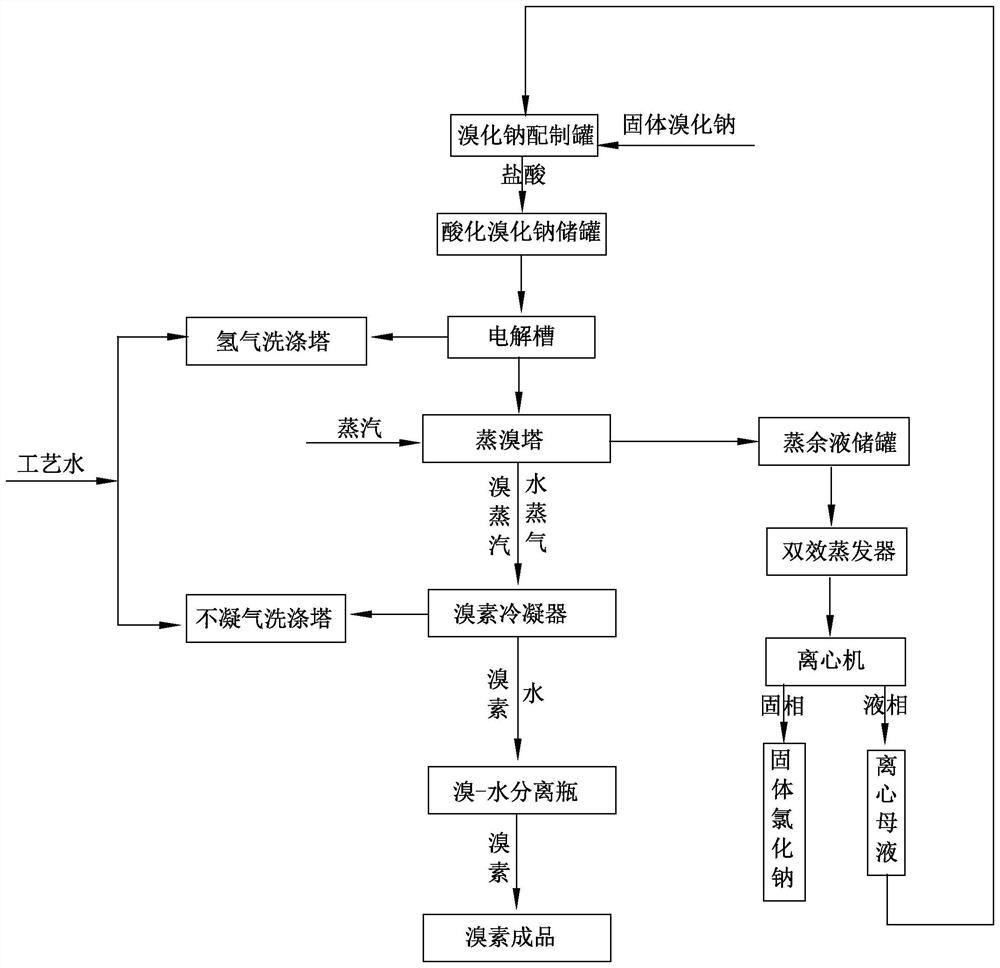
[0059] Such as figure 1 Shown, the process method that electrolytic acidification sodium bromide produces bromine may further comprise the steps:
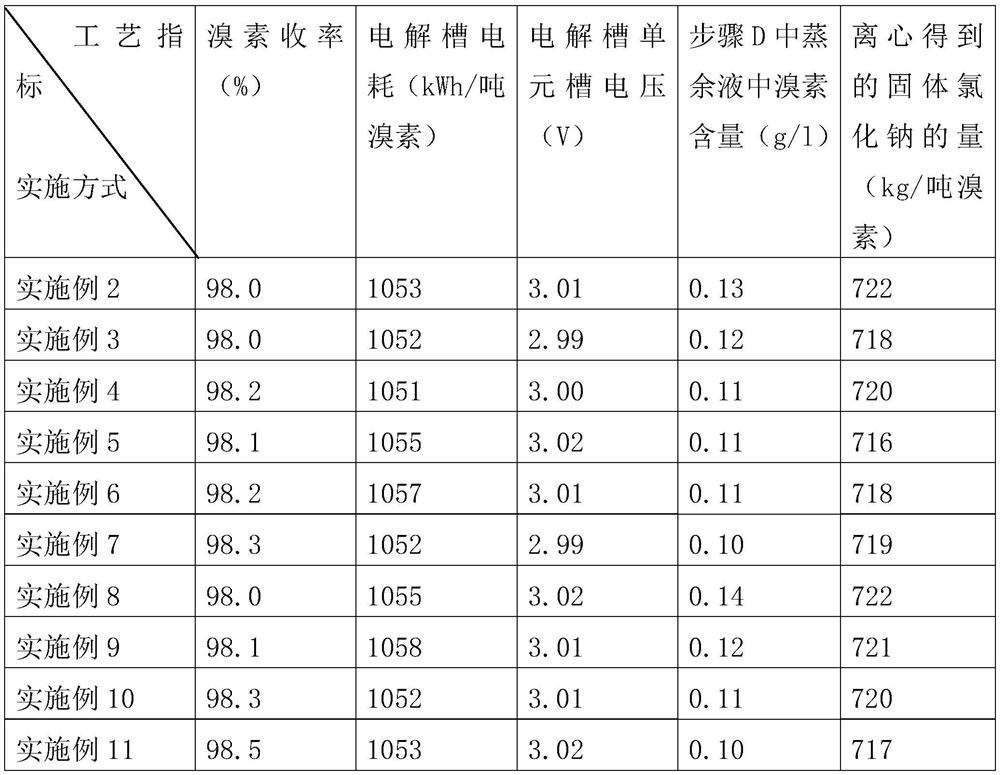
[0060] A: Dissolve solid sodium bromide in water to obtain sodium bromide solution, acidify the sodium bromide solution with hydrochloric acid to obtain acidified sodium bromide solution, the concentration of sodium bromide in the acidified sodium bromide solution is 20%, the concentration of hydrochloric acid 4%.
[0061] B: The acidified sodium bromide solution is pumped into the bottom of the electrolytic cell in Example 1 according to a certain flow rate, and the hydrogen gas produced enters the hydrogen scrubber from the top of the electrolytic cell and is stored after removing a small amount of bromine gas, and the bromine produced The element is dissolved in the acidified sodium bromide solution in the electrolytic cell to form a mixed solution and enters the bromine distillation tower from the top overflow port of the elec...
Embodiment 3
[0073] Such as figure 1 Shown, the process method that electrolytic acidification sodium bromide produces bromine may further comprise the steps:
[0074] A: Dissolve solid sodium bromide in water to obtain sodium bromide solution, acidify the sodium bromide solution with hydrochloric acid to obtain acidified sodium bromide solution, after acidification, the concentration of sodium bromide in the acidified sodium bromide solution is 25%, hydrochloric acid The concentration is 4%.
[0075] B: The acidified sodium bromide solution is pumped into the bottom of the electrolytic cell in Example 1 according to a certain flow rate, and the hydrogen gas produced enters the hydrogen scrubber from the top of the electrolytic cell and is stored after removing a small amount of bromine gas, and the bromine produced The element is dissolved in the acidified sodium bromide solution in the electrolytic cell to form a mixed solution and enters the bromine distillation tower from the top over...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com