Method for simultaneously determining content of various components of traditional Chinese medicine for treating liver diseases
A traditional Chinese medicine, content technology, applied in the direction of measurement device, material separation, analysis of materials, etc., can solve the problems of few components, cumbersome processing, long analysis time, etc., and achieve the effect of saving measurement time, easy operation, and accurate measurement results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] S1. Accurately weigh 4.0 g of traditional Chinese medicine for treating liver diseases with the batch number of 1805311, grind it finely, accurately weigh it, put it in a conical flask, add 25 mL of 50% methanol solution, seal it tightly, weigh it, ultrasonically treat it for 30 minutes, let it cool, and then Weigh the weight, make up the lost weight with 50% methanol solution, shake well, filter through a 0.45 μm microporous membrane, and take the subsequent filtrate to obtain the yellow beetle soft liver solution.
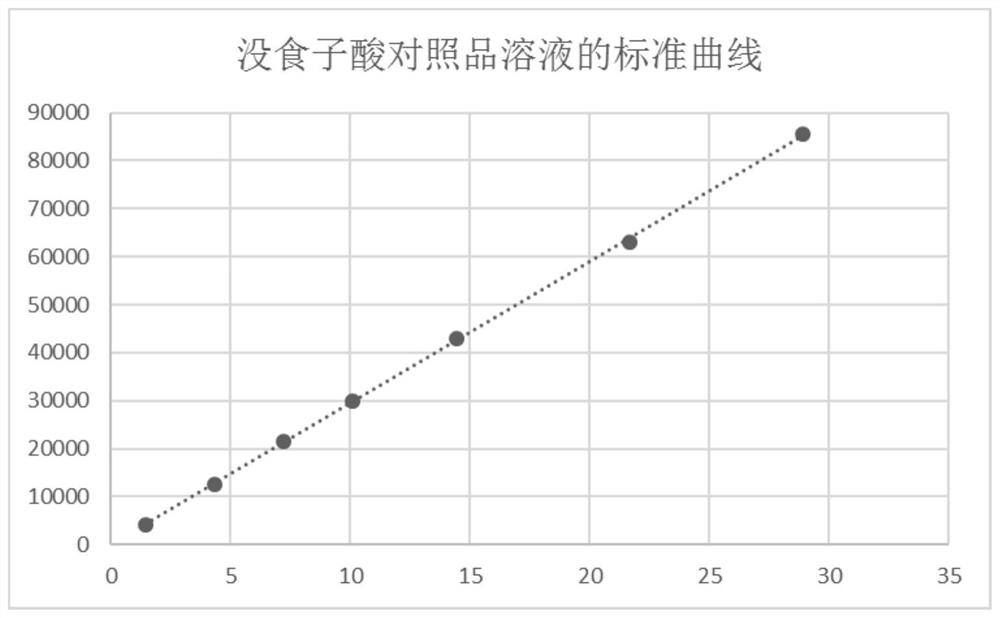
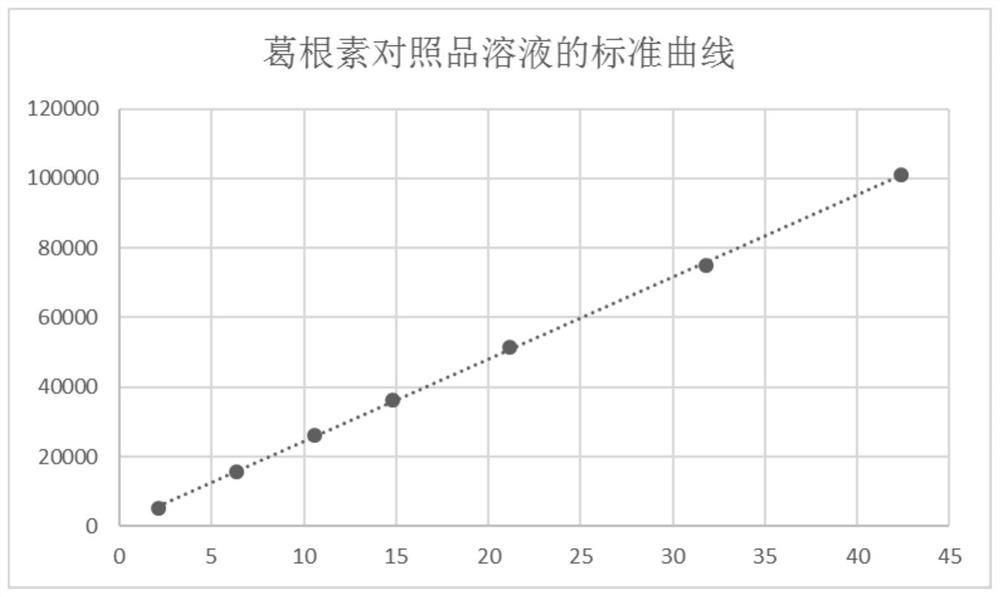
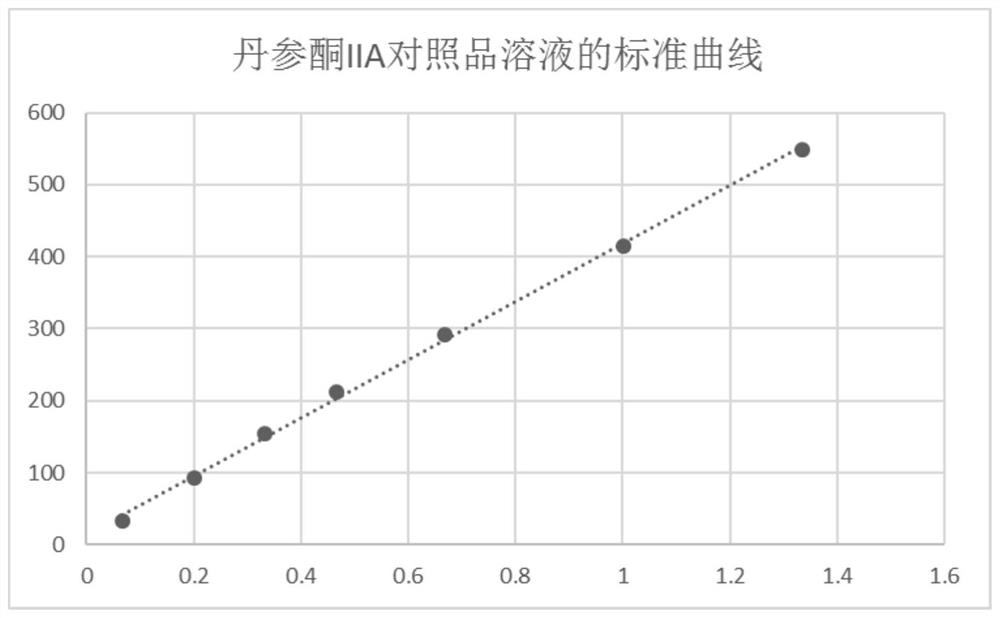
[0079] S2, accurately weigh gallic acid, puerarin, paeoniflorin, notoginseng saponin R 1 , astragaloside IV and tanshinone IIA, add 50% methanol solution to make the reference substance stock solution, accurately draw a certain amount of each reference substance stock solution and put it in the same measuring bottle, add 50% methanol solution to dilute to the mark, and obtain the reference substance solution.
[0080] S3, absorbing the Huangjia Ruangan sol...
Embodiment 2
[0109] Precisely weigh 4g of Chinese medicine for the treatment of liver diseases with batch number 1805312, and measure according to the steps in Example 1. The difference from Example 1 is that the conditions of high performance liquid chromatography are:
[0110] Column: Waters Symmetry C 18 Chromatographic column, specification 250mm*4.6mm, 5μm;
[0111] Mobile phase: mobile phase A is methanol, mobile phase B is 0.08% glacial acetic acid, gradient elution is used, and the mobile phase ratios are all volume percentages:
[0112] 0-25min, mobile phase A is 5%-30%, mobile phase B is 95%-70%;
[0113] 25-50min, mobile phase A is 30%-35%, mobile phase B is 70%-65%;
[0114] 50-65min, mobile phase A is 35%-45%, mobile phase B is 65%-55%;
[0115] 65-95min, mobile phase A is 45%-95%, mobile phase B is 55%-5%;
[0116] 95-105min, mobile phase A is 95%, mobile phase B is 5%;
[0117] Flow rate: 0.8mL / min;
[0118] Column temperature: 25°C;
[0119] Detection wavelength: 270...
Embodiment 3
[0125] Precisely weigh 4g of Chinese medicine for the treatment of liver diseases with batch number 1805313, and measure according to the steps in Example 1. The difference from Example 1 is that the conditions of high performance liquid chromatography are:
[0126] Column: Waters Symmetry C 18 Chromatographic column, specification 250mm*4.6mm, 5μm;
[0127] Mobile phase: mobile phase A is methanol, mobile phase B is 0.12% glacial acetic acid, gradient elution is used, and the mobile phase ratios are all volume percentages:
[0128] 0-25min, mobile phase A is 5%-30%, mobile phase B is 95%-70%;
[0129] 25-50min, mobile phase A is 30%-35%, mobile phase B is 70%-65%;
[0130] 50-65min, mobile phase A is 35%-45%, mobile phase B is 65%-55%;
[0131] 65-95min, mobile phase A is 45%-95%, mobile phase B is 55%-5%;
[0132] 95-105min, mobile phase A is 95%, mobile phase B is 5%;
[0133] Flow rate: 0.9mL / min;
[0134] Column temperature: 27°C;
[0135] Detection wavelength: 270...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com