Preparation method and application of chemical components of anti-cancer active part of rosa laevigata flower
A technology of white wood fragrant flowers and parts, which is applied in the directions of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as unreported chemical ingredients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of PXS66-2:
[0056] A 1.15kg sample of dried flowers of Aquilaria sinensis (Lour.) Spreng. was crushed and extracted with 90% ethanol (2L), ultrasonically extracted in a 60°C water bath for 30min, repeated 4 times, combined and filtered, and the solvent was recovered to obtain an extract . Take 172.0g of the extract, add water (1L) to make a suspension, extract with petroleum ether (1L×3), recover the petroleum ether to obtain the petroleum ether extraction site; the aqueous phase solution after petroleum ether extraction, continue to use ethyl acetate Ester extraction (1L×3), recovery of ethyl acetate to obtain 23.0 g of ethyl acetate extraction fraction (number PXS66-2).
Embodiment 2
[0058] Fractional preparation of PXS66-2 by silica gel column chromatography, and separation of the chemical components of the active segment:
[0059] PXS66-2 with a weight of 23.0 g was eluted with a gradient of dichloromethane-methanol mixed solvent of 1:0, 10:1, 5:1, 3:1, 2:1 and 0:1 through silica gel column chromatography. Divided to give B-1 (5.3g), B-2 (14.0g), B-3 (2.3g), B-4 (0.05g), B-5 (0.08g) and B-6 (0.08g) ) and other 6 parts.
[0060] Take part B-1 5.3g via C 18 Reversed-phase silica gel column chromatography, 5:95→100:0 methanol-water gradient elution to obtain B-1-1 (0.05 g), B-1-2 (0.11 g), B-1-3 (0.09 g), B-1-4 (0.08g), B-1-5 (0.07g), B-1-6 (1.12g), B-1-7 (0.89g), B-1-8 (0.18 g), 12 parts including B-1-9 (0.17g), B-1-10 (0.12g), B-1-11 (0.11g) and B-1-12 (0.03g).
[0061]1.12 g of part B-1-6 was taken, and washed with a gradient of petroleum ether-ethyl acetate mixed solvent of 5:1, 4:1, 3:1, 2:1, 1:1 and 0:1 through silica gel column chromatography ...
Embodiment 3
[0063] Spectral data and structural analysis of the new compound aquilarolide A (PFS-11):
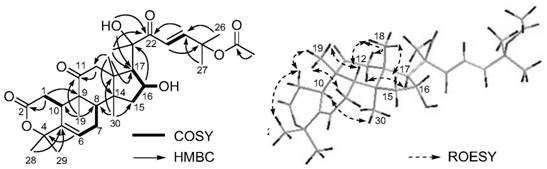
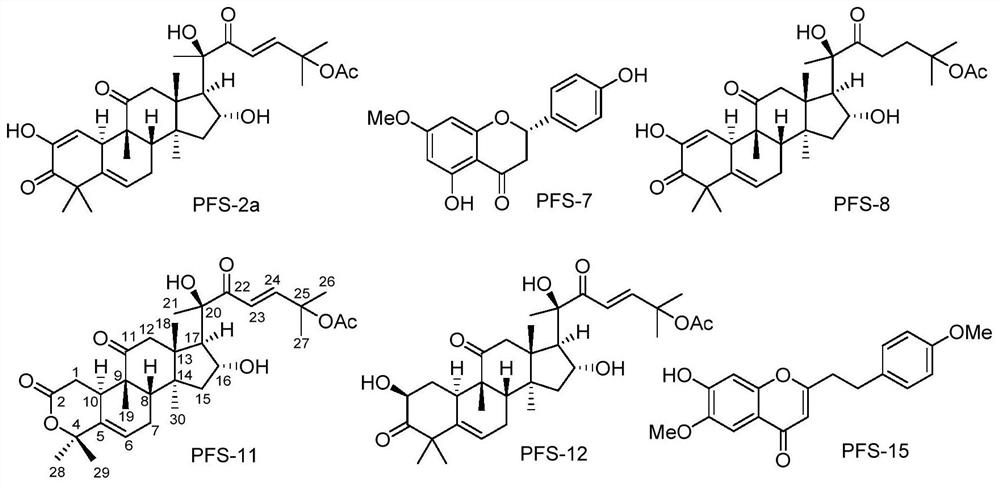
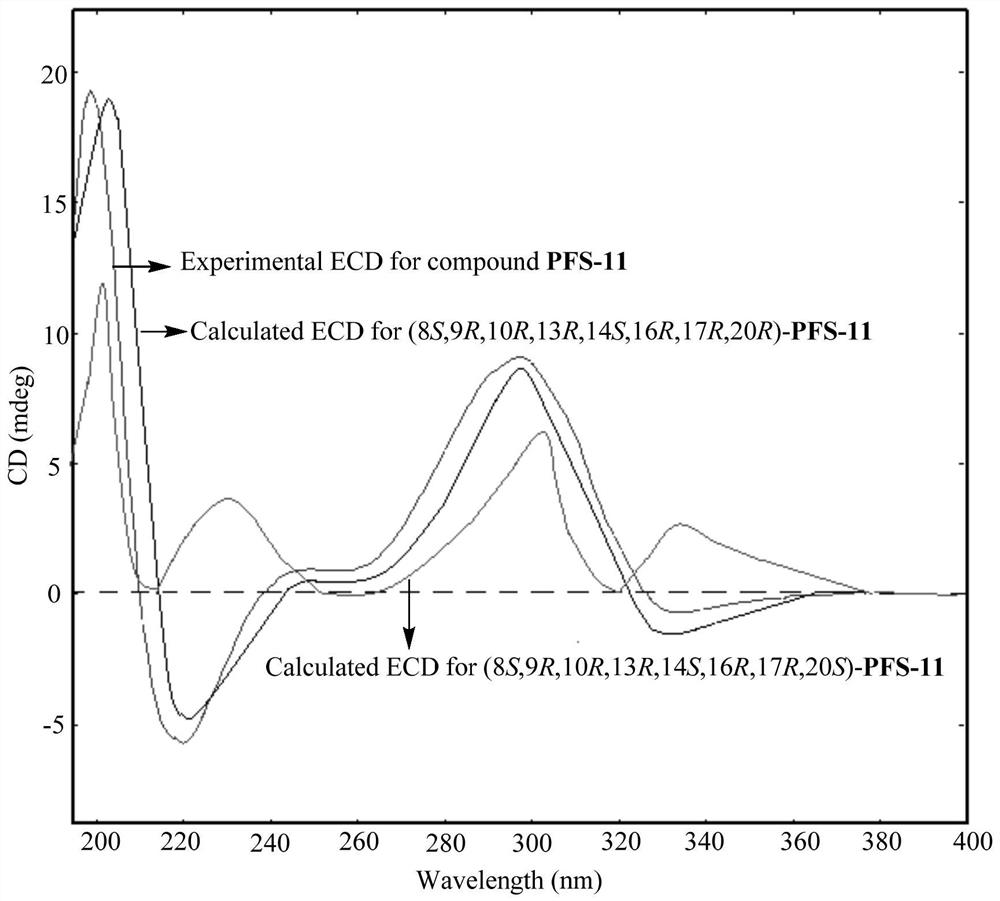
[0064] According to the high-resolution mass spectrometry data of compound PFS-11, HR-ESI-MS m / z 567.2937[M+Na] + (calcd.for C 31 H 44 NaO 8 , 567.2934), inferred that the molecular formula of PFS-11 is C 31 H 44 O 8 , with 10 degrees of unsaturation. The nuclear magnetic resonance (NMR) data (Table 1) of this compound were compared with those of cucurbitane-type triterpenes, especially with the NMR data of the compound Neocucurbitacin D [Jiang H-Z, Hu S, Tan R-X, Tan R, Jiao R-H. Neocucurbitacin D, a novel lactone-type norcucurbitacin as xanthine oxidaseinhibitor from Herpetospermum pedunculosum. Natural Product Research, 2020, 34, 1728-1734], it is speculated that PFS-11 is a norcucurbitane triterpenoid lactone. The chemical structural formula of PFS-11 was finally determined by the two-dimensional NMR correlation map ( figure 2 ) analysis and electronic circular dichroism ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com