Kidney-targeting DNA nanometer raft-IL-33 as well as preparation method and application thereof
A kidney-targeted, SH-DNA technology, applied in the field of kidney-targeted DNA nano-raft-IL-33 and its preparation, can solve problems such as complexity, poor accumulation, poor cytokine targeting performance, etc. Achieve the effects of relieving pain, reducing drug costs, and improving drug targeting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The invention discloses a method for synthesizing cytokine drugs based on DNA nano rafts, which comprises the following steps:
[0038] (1) Preparation: Prepare DNA single strands (M 13mp 18DNA single strands, staple strands, capture strands), thiol-modified DNA (SH-DNA), recombinant interleukin 33, 3-(2-pyridyl dithio) N-Hydroxysuccinimide Propionate (SPDP), 1xTAE-Mg 2+ buffer.
[0039] (2) The preparation of DNA-IL-33 was separated and purified by ultrafiltration, and the preparation efficiency was characterized by ultraviolet-visible mass spectroscopy;
[0040] (3) Synthesis of DNA origami rafts, by PCR synthesizing a capture strand complementary to the DNA in step (1). It was separated and purified by ultrafiltration, and its morphology was characterized by atomic force microscopy (AFM).
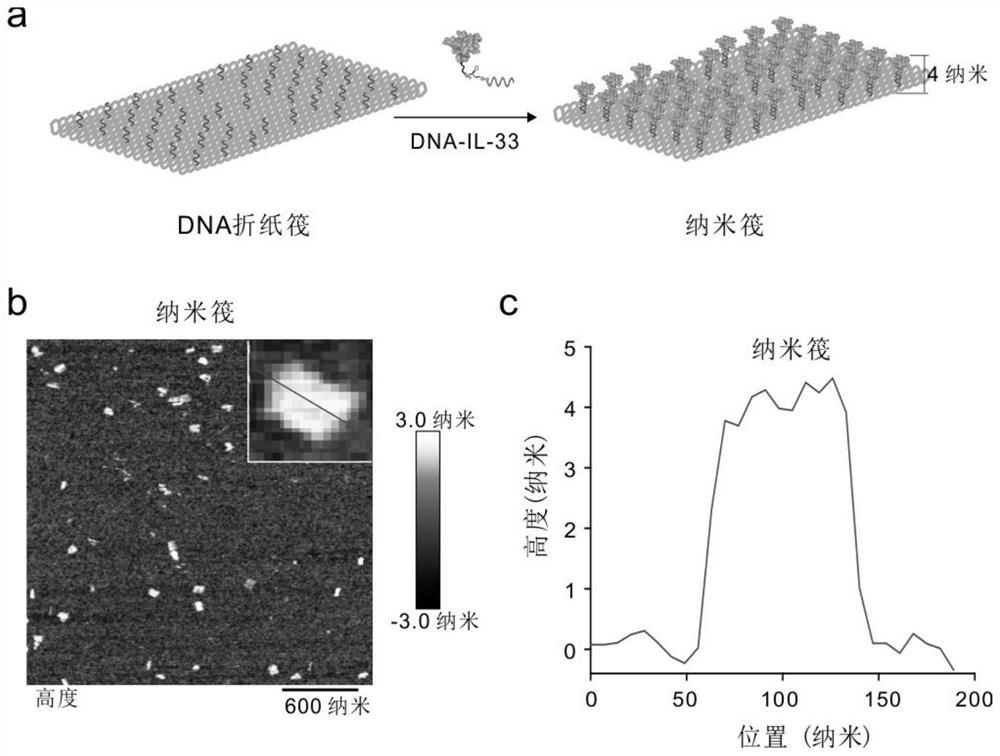
[0041] (4) The assembly and synthesis of DNA nano-rafts, the DNA-IL-33 in step (1) and the DNA origami rafts in step (2) were mixed, assembled and synthesized DNA nano-rafts, s...
Embodiment 1
[0062] Example 1 Preparation of DNA-IL-33
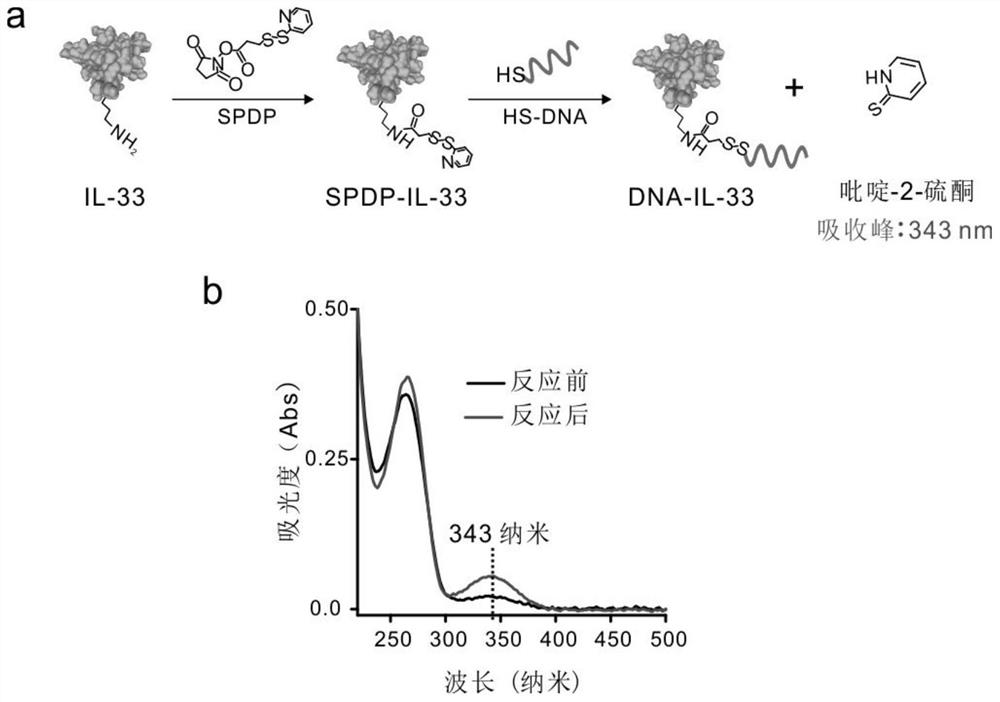
[0063] Dissolve 100 μg of IL-33 powder in 50 μL of NaCl solution. React 50 μL of 6.6 μM IL-33 solution with 50-fold excess of SPDP in 1x PBS buffer (pH 8.5) for 2 hours. Excess SPDP was removed by Zeba spin desalting column (7KMWCO, 0.5 mL). Then, IL-33 was coupled to sulfhydryl-modified DNA strands (3-fold excess) in 1×PBS buffer (pH 7.4) for 8 hours at room temperature. The efficiency of HS-DNA modification of IL-33 was assessed by monitoring the net increase in UV-vis spectrum at 343 nm due to the generation of pyridine-2-thione (extinction coefficient: 8080 M-1 cm-1). In 1x PBS (pH=7.4), ultrafiltration (3000 g, 10 minutes) was performed 3 times with a 30 kD cut-off filter membrane (Amicon) to remove excess SH-DNA strands.
Embodiment 2
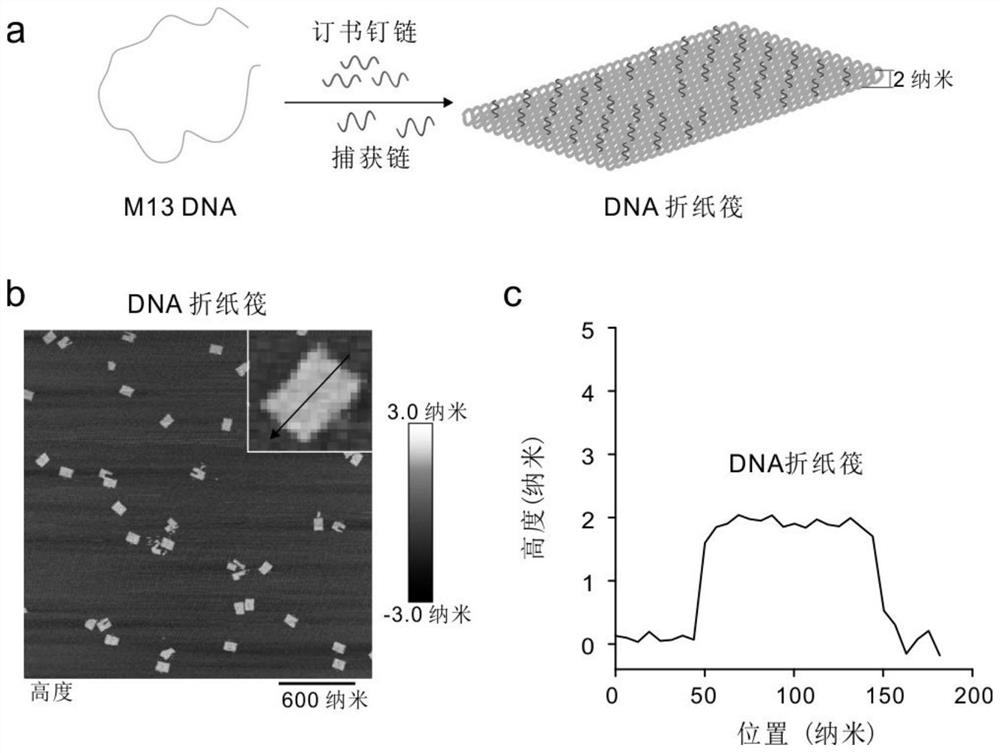
[0064] Example 2 Synthesis of DNA origami rafts
[0065] In 1x TAE-Mg 2+ In buffer, 10 nM of M 13mp 18 DNA single strand was mixed with 50-fold excess of staple strand and 100-fold excess of capture. The mixture was annealed from 95 °C to 4 °C.
[0066] Table 3 Annealing program from 95℃ to 4℃
[0067] temperature gradient 95℃ 2min 95-60℃ -0.1℃ / 12s 60℃ 12min 60-25℃ -0.1℃ / 12s 4℃ Keep
[0068] In 1xTAE-Mg 2+ Excess staple strands were removed by ultrafiltration (3000g, 10min) twice in buffer and once (3000g, 10min) in 1x PBS (pH=7.4) and capture chains, and were characterized by atomic force microscopy (AFM).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com