Preparation method of medetomidine
A technology of medetomidine and trimethylsilimidazole, which is applied in the field of drug synthesis, can solve the problems of difficult recycling, large amount of catalyst, and difficult recovery of solvents, and achieve the effect of reducing solvent loss and emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
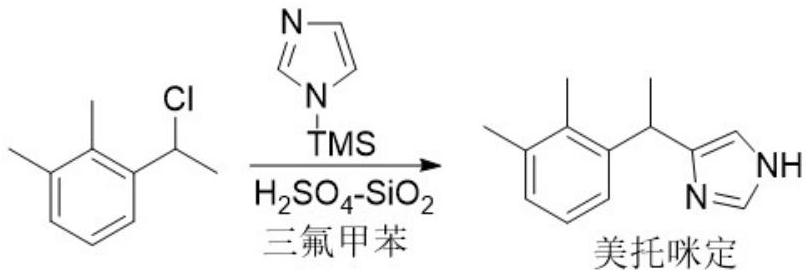
[0022]
[0023] 100 g of trifluorotoluene and 1.68 g of sulfuric acid-silica gel (prepared by referring to the method of Tetrahedron Letters, 2007, 48, 3783-3787) were successively put into the reaction flask equipped with a mechanical stirrer, a built-in thermometer, and a dropping funnel, and the internal temperature was lowered. to -10°C, then add dropwise a solution consisting of 16.83g N-trimethylsilimidazole and 100g trifluorotoluene at this temperature, and stir for 30 minutes after the addition; slowly dropwise add 16.87g 1-(1-chloroethyl) The solution composed of -2,3-xylene and 136g trifluorotoluene, at this time, the internal temperature is controlled at -10~-5°C. After the dropwise addition is completed, continue to stir at this temperature for 4 hours, and thin-layer chromatography (TLC ) to monitor the reaction process; after the reaction was completed, vacuum filtration was performed, and the filter cake was rinsed with 10g trifluorotoluene. The filter cake (...
Embodiment 2
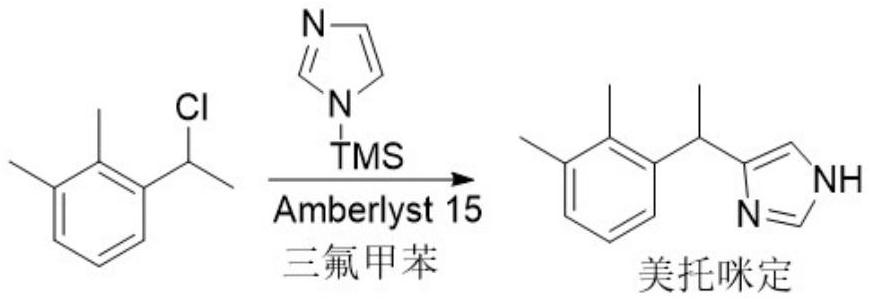
[0027]
[0028] Add 100 g of trifluorotoluene and 2.81 g of Amberlyst 15 cation exchange resin to the reaction flask equipped with a mechanical stirrer, a built-in thermometer, and a dropping funnel, and drop the internal temperature to -5°C, then dropwise add 16.83 The solution composed of gN-trimethylsilimidazole and 100g trifluorotoluene was stirred for 30min after the addition was completed. Slowly add dropwise a solution consisting of 16.87g 1-(1-chloroethyl)-2,3-xylene and 136g trifluorotoluene. At this time, control the internal temperature at -5~0°C. Continue stirring for 7 hours at high temperature, and monitor the reaction process with thin-layer chromatography (TLC); after the reaction is completed, filter under reduced pressure, and rinse the filter cake with 10g trifluorotoluene, and the filter cake (catalyst) is naturally dried for subsequent use Filtrate is heated to 50 ℃ and is incubated 1 hour, after natural cooling, water 30ml * 3, saturated saline 30ml wa...
Embodiment 3
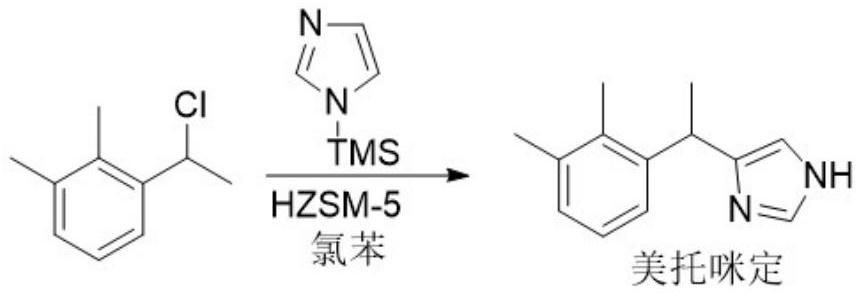
[0031]
[0032] Put 100g of chlorobenzene and 3g of HZSM-5 molecular sieve into the reaction flask equipped with a mechanical stirrer, a built-in thermometer and a dropping funnel successively, lower the internal temperature to 0°C, and then add dropwise 16.83g of N-trimethyl A solution composed of silyl imidazole and 100g chlorobenzene was stirred for 30min after the addition; a solution composed of 16.87g1-(1-chloroethyl)-2,3-xylene and 136g chlorobenzene was slowly added dropwise. temperature does not exceed 5°C, after the dropwise addition is completed, continue to stir at this temperature for 8 hours, and monitor the reaction process with thin-layer chromatography (TLC); Cake, filter cake (catalyst) was rinsed with a small amount of ether and dried naturally for later use; the filtrate was heated to 50°C and kept for 1 hour, and after natural cooling, it was washed with 30ml×3 water and 30ml saturated saline, and the organic layer was washed with anhydrous sodium sulfat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com