Preparation method of gabapentin acetate
A technology of ganirelix and acetic acid, applied in the field of polypeptide drug production, can solve the problems of long reaction time, large environmental pollution and low yield, and achieve the effect of long reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
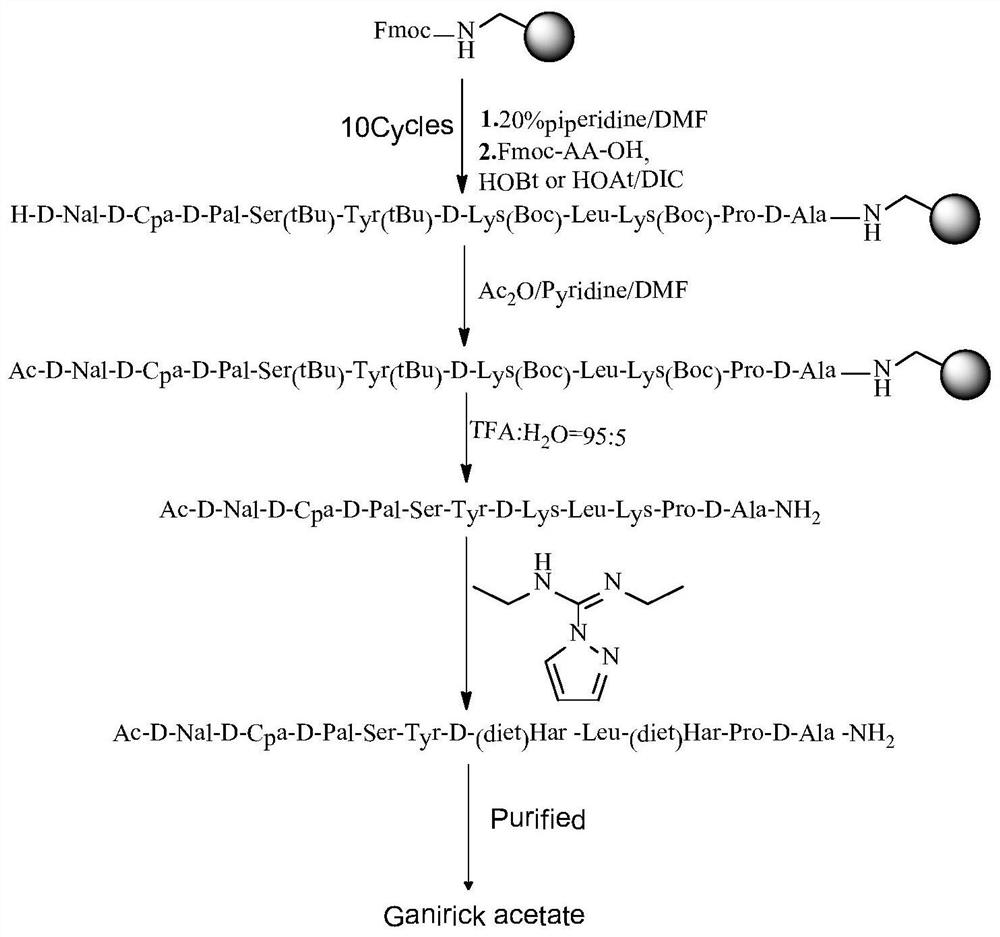
[0053] Embodiment 1: Amino resin is coupled with amino acid to obtain linear peptide peptide resin
[0054] Put 53.5g of Rink Amide resin with a substitution degree of 0.6mmol / g into the solid-phase reaction column, swell the amino resin with DMF for half an hour, add DBLK to deprotect it for 5min+7min, wash the resin 6 times with DMF, and the ninhydrin detection resin shows that the result is positive.
[0055] Weigh Fmoc-D-Ala-OH (30.0g, 96.4mmol) and HOBt (14.3g, 106.04mmol) and dissolve them in DMF, add DIC (17.9mL, 115.6mmol) to activate for 5min, then add the mixture to the reaction column , reacted at room temperature for 2 hours, and the ninhydrin test was negative. Wash the resin 3 times with DMF, add DBLK to deprotect for 5min + 7min, wash the resin 6 times with DMF, and the ninhydrin test result is positive.
[0056] Repeat the above steps to complete Fmoc-Pro-OH, Fmoc-Lys(Boc)-OH, Fmoc-Leu-OH, Fmoc-D-Lys(Boc)-OH, Fmoc-Tyr(tBu) sequentially by coupling one by one ...
Embodiment 2
[0057] Example 2: Acetylation of Peptide Resins
[0058] After the amino acid coupling was completed, DBLK was added for deprotection for 5min+7min, and the resin was washed with DMF for 6 times. The ninhydrin test was positive. Weigh 60.2ml of acetic anhydride and 51.7ml of pyridine and dilute with DMF to 150ml, put them into the solid-phase reaction column and react at room temperature for 2 hours. After the reaction is completed, the ninhydrin test is negative, wash with DMF for 6 times, shrink with methanol for 3 times, and vacuum dry 124.3 g of peptide resin was obtained.
Embodiment 3
[0059] Example 3: Cleavage to obtain linear peptides
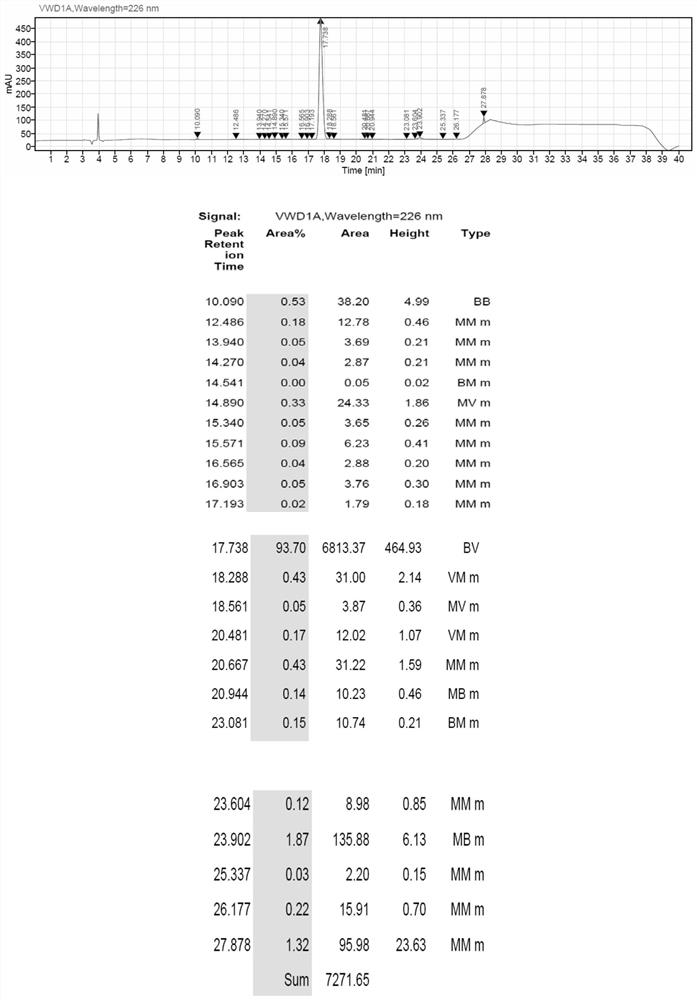
[0060] Add the above-mentioned peptide resin into a 2L flask, and add the lysis reagent (TFA:H 2 O (volume ratio)=95:5, 992mL), stirred at room temperature for 2.5 hours, filtered the resin, and collected the filtrate. The resin was washed with a small amount of TFA, and the filtrates were combined. The filtrate was slowly added to 10 L of glacial ether for precipitation. Centrifuged, washed twice with ether, and dried with nitrogen gas to obtain 62.86 g of linear peptide, the purity of linear peptide was 93.70%, and the yield was 97.2%.
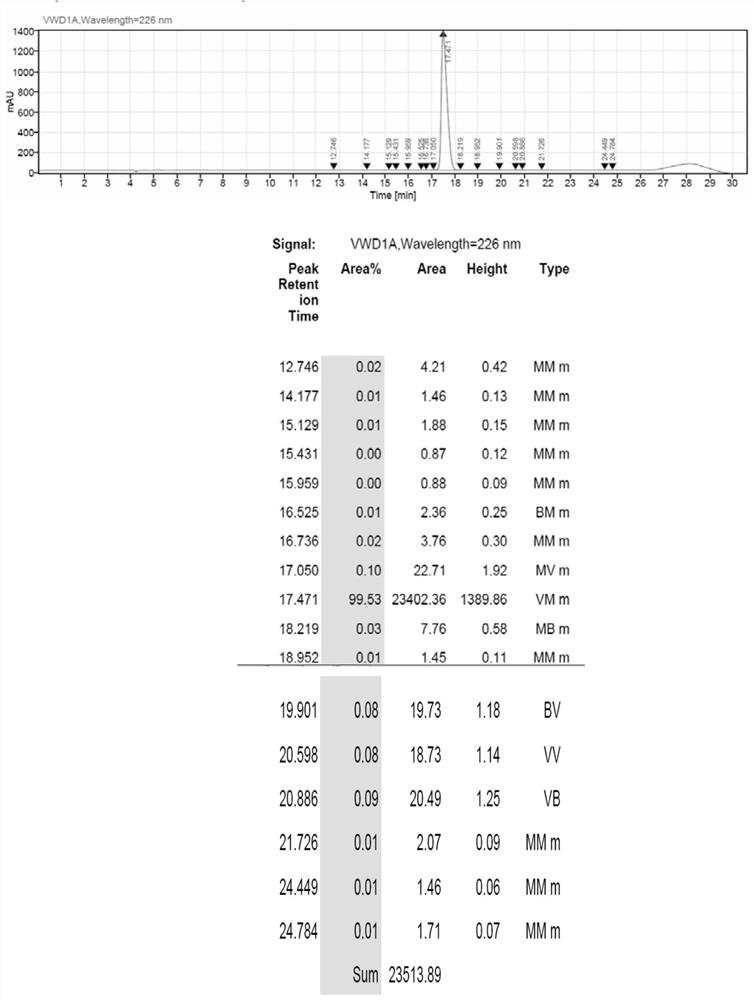
[0061] After detection, the linear peptide MS data of Ganirelix acetate is: 1373.561 (M+1); 1395.632 (M+1), and the HPLC spectrum is as follows figure 2 As shown, wherein the retention value T=17.738 is the target peak of the product, and the purity is 93.70%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com