Novel method for synthesizing 2-chloro-5-aminophenol
The technology of an aminophenol and a new method is applied in the directions of chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., and can solve the problems of high comprehensive cost, complicated process, and many kinds of raw materials, etc., and achieves easy separation and purification of products, The effect of simple post-treatment process and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
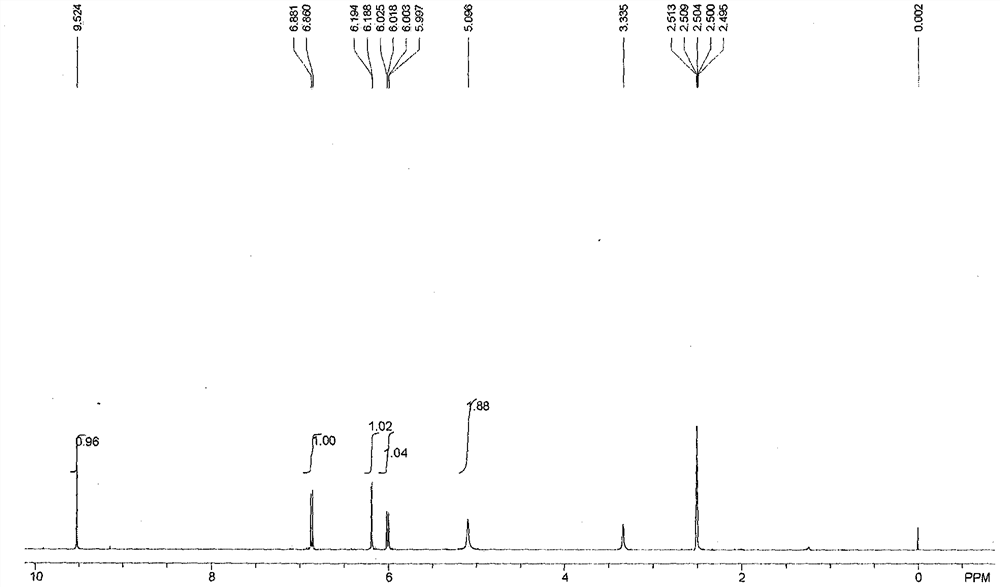
[0031] Add 110 kg of m-aminophenol in a 5000 liter reactor, pump 1000 liters of water, stir and dissolve; add 135 kg of N-chlorosuccinimide in another 2000 liter reactor, pump 1000 liters of ethanol, stir Make it dissolve; slowly pump the ethanol solution of N-chlorosuccinimide into the above-mentioned 5000-liter reaction kettle through a metering pump, and control the temperature at 45-50 ° C. When the ethanol solution of N-chlorosuccinimide The solution is added, and the liquid chromatography monitors the reaction process. When the m-aminophenol content is lower than 0.1%, the reaction is stopped, and the ethanol and most of the water are evaporated under reduced pressure. The residue is cooled to room temperature, and 200 liters of ice water are slowly added under stirring. Immediately a large amount of yellow precipitate was produced, continued to stir for 1 hour, centrifuged, the solid filter cake was washed with 100 liters of cold water, and dried to obtain 71.5 kg of 2-c...
Embodiment 2
[0033] Add 110 kg of m-aminophenol in a 5000 liter reactor, pump 1000 liters of N, N-dimethylformamide, stir and dissolve; add 160 kg of N-chlorosuccinyl in another 2000 liter reactor Amine, pump 1000 liters of N, N-dimethylformamide, stir to dissolve it; slowly pump the N, N-dimethylformamide solution of N-chlorosuccinimide into the above 5000 In a liter reaction kettle, under stirring, the temperature is controlled at 55-65°C. When the N,N-dimethylformamide solution of N-chlorosuccinimide is added, the reaction process is monitored by liquid chromatography. When the phenol content is lower than 0.1%, stop the reaction, distill out N,N-dimethylformamide under reduced pressure, cool the residue to room temperature, slowly add 300 liters of ice water under stirring, immediately a large amount of yellow precipitate is produced, continue stirring Centrifuge for 1 hour, wash the solid filter cake with 100 liters of cold water, and dry to obtain 93 kg of 2-chloro-5-aminophenol with...
Embodiment 3
[0035] Add 110 kilograms of m-aminophenol in 5000 liters of reactor, pump into 1000 liters of tetrahydrofuran, stir and dissolve; add 160 kilograms of N-chlorosuccinimide in another 2000 liters of reactor, pump into 1000 liters of tetrahydrofuran, Stir to dissolve it, and control the temperature at 50°C-60°C; slowly pump the tetrahydrofuran solution of N-chlorosuccinimide into the above-mentioned 5000-liter reaction kettle through a metering pump, when N-chlorosuccinimide Add the tetrahydrofuran solution of the amine, finish adding, reflux for 2 hours, monitor the reaction process by liquid chromatography, stop the reaction when the content of m-aminophenol is lower than 0.1%, distill off the tetrahydrofuran under reduced pressure, cool the residue to room temperature, and slowly Add 300 liters of ice water, a large amount of yellow precipitate is produced immediately, continue to stir for 1 hour, centrifuge, the solid filter cake is washed with 100 liters of cold water, dried ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com