Preparation method and application of porous polymer containing iron and boron
A porous polymer, iron source technology, applied in electrodes, electrolysis processes, electrolysis components, etc., can solve problems such as instability and achieve the effect of enriching the pore structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of the porous polymer containing iron and boron in this embodiment is as follows:
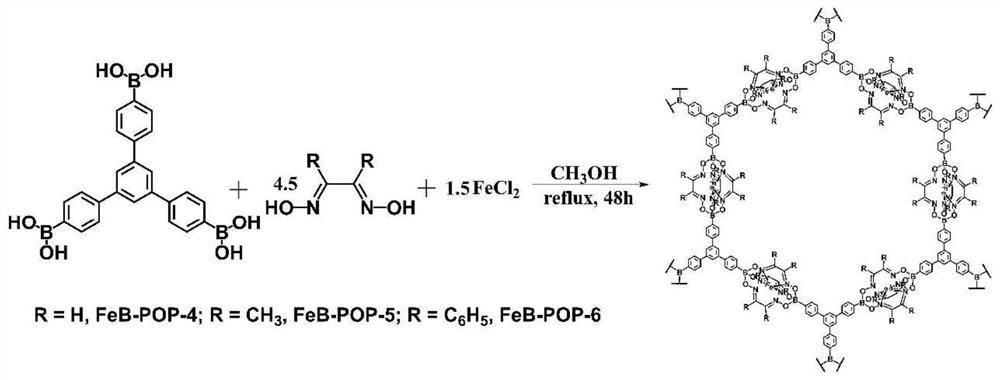
[0036] (1) 0.3 mmol of boron-containing tetrahedral building block tetrakis (4-boronic acid phenyl), 1.2 mmol of ferrous chloride and 1.8 mmol of 2,6-diformyl-4-methylphenol di Oxime (synthetic schematic diagram see Figure 6 ) into 50mL of methanol solvent, stirring and dispersing evenly.
[0037] (2) Stir and disperse evenly and put it into a round bottom flask.
[0038] (3) The round-bottomed flask was refluxed and stirred at 65° C. for 48 hours, cooled to room temperature after the reaction, and the solid was collected by suction filtration.
[0039] (4) The collected solid was centrifuged and washed 10 mL×5 times with DMF and THF successively, and the solid was collected again.
[0040] (5) After Soxhlet extraction of the solid with THF for 24 hours, vacuum drying at 90° C. for 8 hours to obtain a black solid, which is the porous polymer containing iron and...
Embodiment 2
[0047] The preparation method of the porous polymer containing iron and boron in this embodiment is as follows:
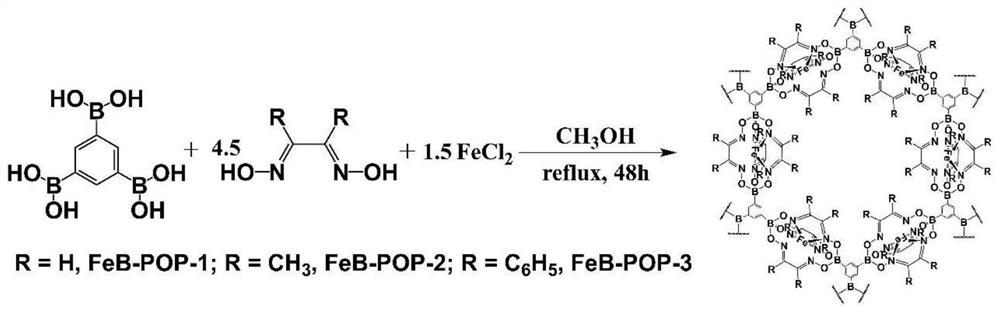
[0048] (1) 0.3mmol of benzene-1,3,5-triyltriboric acid, 0.45mmol of ferrous chloride and 1.35mmol of glyoxime (see figure 1 ) into 55mL of methanol solvent, stirring and dispersing evenly.
[0049] (2) Stir and disperse evenly and put it into a round bottom flask.
[0050] (3) The round-bottomed flask was refluxed and stirred at 60° C. for 42 hours, cooled to room temperature after the reaction, and the solid was collected by suction filtration.
[0051] (4) The collected solid was centrifuged and washed 10 mL×5 times with DMF and THF successively, and the solid was collected again.
[0052] (5) After Soxhlet extraction of the solid THF for 24 hours, vacuum drying at 90° C. for 8 hours to obtain a black solid, which is the porous polymer containing iron and boron, marked as FeB-POP-1.
[0053] The same method as in Example 1 was used to carry out the electrocataly...
Embodiment 3
[0055] The preparation method of the porous polymer containing iron and boron in this embodiment is as follows:
[0056] (1) 0.3mmol of benzene-1,3,5-triyltriboric acid, 0.45mmol of ferrous chloride and 1.35mmol of dimethylglyoxaloxime (synthetic schematic diagram see figure 1 ) into 50mL of methanol solvent, stirring and dispersing evenly.
[0057] (2) Stir and disperse evenly and put it into a round bottom flask.
[0058] (3) The round-bottomed flask was refluxed and stirred at 60° C. for 40 hours at a constant temperature. After the reaction was completed, it was cooled to room temperature and the solid was collected by suction filtration.
[0059] (4) The collected solid was centrifuged and washed 20 mL×6 times with DMF and THF successively, and the solid was collected again.
[0060] (5) After Soxhlet extraction of solid THF for 24 hours, vacuum drying at 85° C. for 24 hours to obtain a black powder, which is the porous polymer containing iron and boron, marked as FeB-P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com