Method for separating mixed salt of potassium fluoride, potassium bromide, potassium carbonate and potassium bicarbonate
A technology of potassium bicarbonate and potassium bromide, which is applied in the field of potassium fluoride mixed salt, potassium bicarbonate, potassium bromide, and potassium carbonate separation, can solve problems such as complex composition, high processing cost, and inability to realize resource utilization, and achieve Reduce pollution and hazards, achieve the effect of resource recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
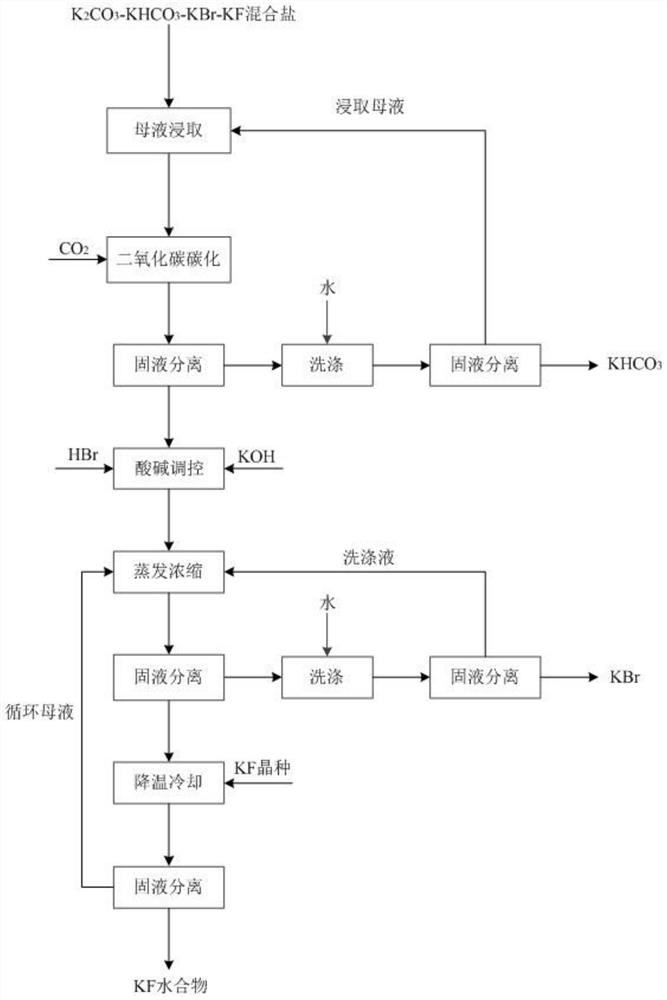
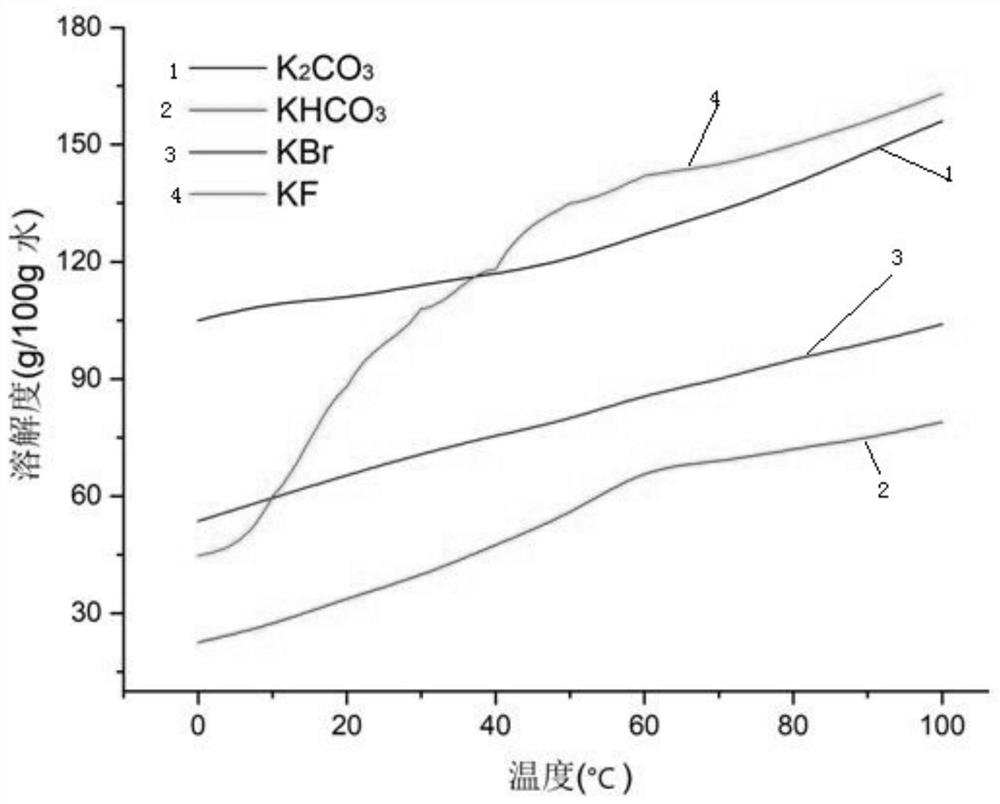
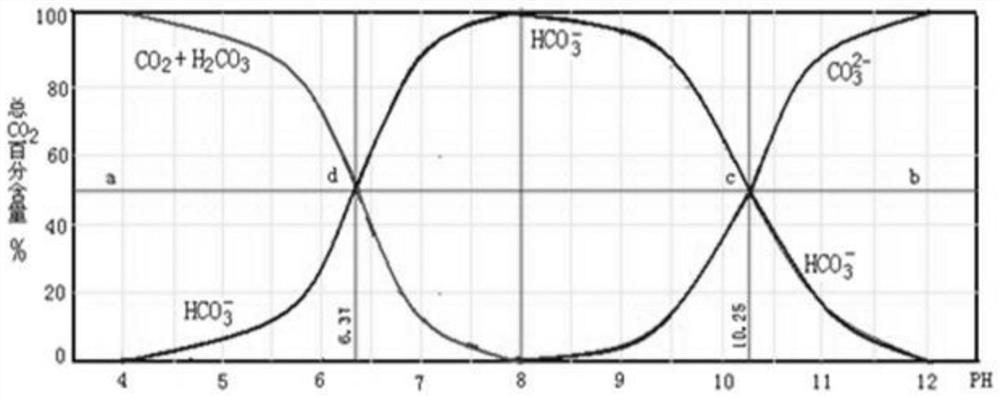
[0082] Get 1kg of potassium carbonate, potassium bicarbonate, potassium bromide, and potassium fluoride mixed salt, add the leaching mother liquor (saturated potassium bicarbonate aqueous solution) and stir evenly, then introduce carbon dioxide, the pH of the salt solution drops to 7.8 for solid-liquid separation, and obtain carbonic acid Potassium hydrogen crude salt and filtrate. Potassium bicarbonate crude salt is washed with water, and the amount of water added is 1.5 times the quality of potassium fluoride, potassium bromide, potassium carbonate, and potassium bicarbonate mixed salt. After washing is completed, solid-liquid separation is carried out. The obtained solid is potassium bicarbonate product, and the obtained solution Return to the leaching step to serve as the leaching mother liquor; add hydrobromic acid to the filtrate to make the pH drop to 3.6, and then add potassium hydroxide to make the pH of the solution 7.3. The obtained neutral solution is heated to 121...
Embodiment 2
[0084] Take 1 kg of potassium carbonate, potassium bicarbonate, potassium bromide, and potassium fluoride mixed salt, add the leaching mother liquor and stir evenly, then pass in carbon dioxide, and the pH of the salt solution drops to 7.5 for solid-liquid separation to obtain potassium bicarbonate coarse salt and filtrate. Potassium bicarbonate coarse salt is washed with water, and the amount of water added is 1.2 times the quality of potassium fluoride, potassium bromide, potassium carbonate, and potassium bicarbonate mixed salt. After washing, solid-liquid separation is carried out. The obtained solid is potassium bicarbonate product, and the obtained solution Return to the leaching step to serve as the leaching mother liquor; hydrobromic acid is added to the filtrate to make the pH drop to 3.8, and then potassium hydroxide is added to make the pH of the solution 6.8. The obtained neutral solution is heated to 123° C. for evaporation and concentration, and after heat preserv...
Embodiment 3
[0086] Take 1 kg of mixed salt of potassium carbonate, potassium bicarbonate, potassium bromide, and potassium fluoride, add the leaching mother liquor and stir evenly, then pass in carbon dioxide, and the pH of the salt solution drops to 7.3 for solid-liquid separation to obtain potassium bicarbonate coarse salt and filtrate. Potassium bicarbonate crude salt is washed with water, the amount of water added is 1 time of the quality of potassium fluoride, potassium bromide, potassium carbonate, potassium bicarbonate mixed salt, after washing is completed, solid-liquid separation is carried out, the obtained solid is potassium bicarbonate product, and the obtained solution Return to the leaching step to serve as the leaching mother liquor; hydrobromic acid is added to the filtrate to reduce its pH to 3, and then potassium hydroxide is added to make the pH of the solution 7.5. The obtained neutral solution is heated to 122° C. for evaporation and concentration, and after heat prese...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com