Non-noble metal copper-based catalyst and application thereof in benzylamine oxidative coupling reaction
A technology of copper-based catalysts and non-noble metals, applied in catalytic reactions, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve high reaction temperature, large amount of metal, and large amount of reaction solvent and other problems, to achieve the effect of simple preparation and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
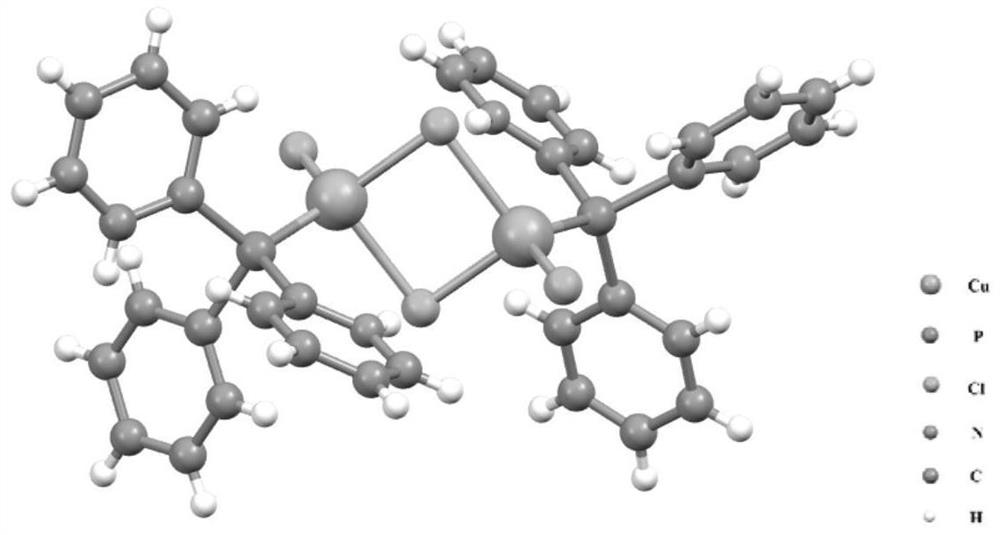
[0024] Example 1: Cu 2 Preparation of complexes
[0025] Put copper chloride (40 mg, 0.2 mmol) into a round bottom flask, then add 15 mL of methanol and 5 mL of N,N-dimethylformamide, stir for 5 minutes, then add diphenyl-2-pyridylphosphine ( 80mg, 0.3mmol), the solution turns dark green; continue to stir for 15min, add NaBH containing NaBH to the above solution 4 (20mg, 0.52mmol) in 0.5mL deionized water, the solution turned yellow. Stirring was continued for 9 hours and the stirring was stopped. After the reaction stopped, the aqueous phase was removed, followed by deionized water and CH 2 Cl 2 Wash the organic phase several times. The dichloromethane phase was recovered, and the obtained dichloromethane solution was spin-dried. Solid with a small amount of CH 2 Cl 2 The solution is dissolved, and it is stored in a single crystal bottle, and the upper layer of the single crystal bottle is covered with n-hexane solution (dichloromethane: normal hexane volume ratio is ...
Embodiment 2
[0026] Example 2: Cu 2 / γ-Al 2 o 3 preparation of
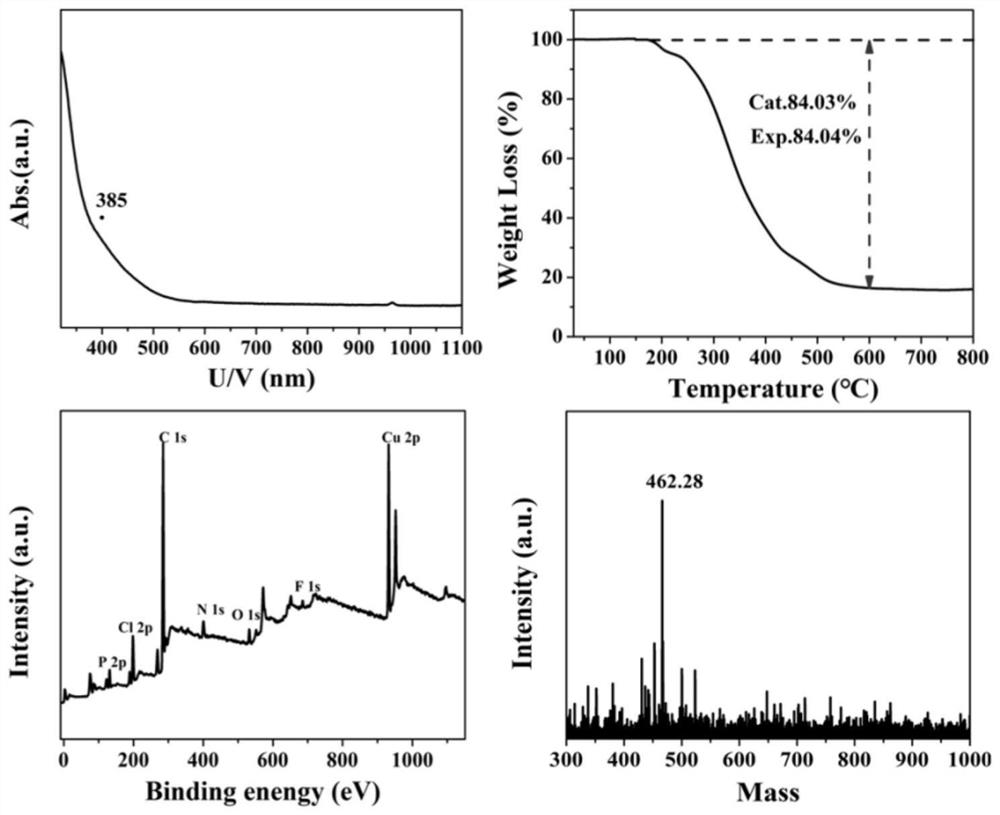
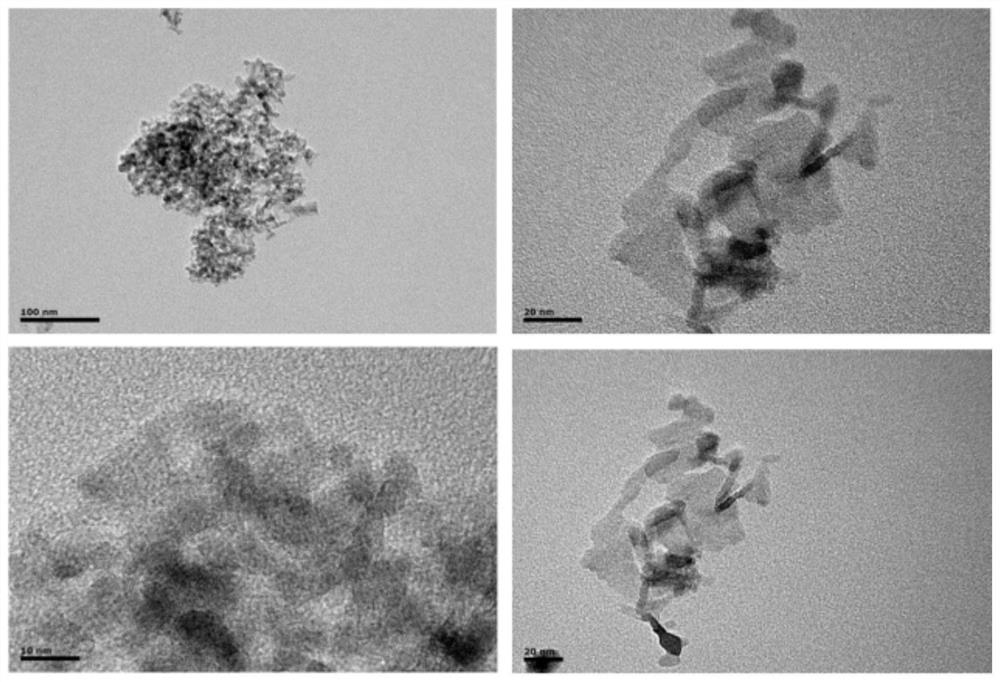
[0027] γ-Al 2 o 3 (100mg) was dissolved in a mixed solution of 15mL methanol and 5mL dichloromethane, then Cu 2 The complex (1.2 mg) was dissolved in N,N-dimethylformamide (0.5 mL), which was added dropwise to a mixed solution of methanol and dichloromethane. After stirring for 6 hours, the stirring was stopped. The product was collected by centrifugation (10000 rpm) and washed twice with methanol. Finally, the solid was dried in a vacuum oven at 50 °C overnight, and the solid was collected to obtain Cu 2 / γ-Al2 o 3 catalyst. attached image 3 for Cu 2 / γ-Al 2 o 3 TEM image, it can be seen from the image that Cu 2 / γ-Al 2 o 3 It is in the form of nano flakes, and there are no metal particles on the surface.
Embodiment 3
[0028] Example 3: γ-Al 2 o 3 Catalyzes the oxidative coupling reaction of benzylamine.
[0029] Add 0.46mmol benzylamine and 30mgγ-Al to a 10mL Schlenk reaction flask in sequence 2 o 3 Catalyst, 100 microliters of tert-butanol hydroperoxide (TBHP) and 1 mL of acetonitrile, stirred and reacted at 50°C for 13h, after the reaction was completed, the temperature of the reaction solution was cooled to room temperature, and the solid and liquid were separated by centrifugation (10000rpm), and the reaction solution was passed through According to GC analysis, the yield was 27.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com