Preparation method and application of universal chimeric antigen receptor regulatory T cell
A chimeric antigen receptor and cell technology, which is applied in the field of general chimeric antigen receptor regulatory T cell preparation, can solve problems such as death and organ damage of patients, and achieve the effect of inhibiting inflammatory reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0032] see Figure 1 to Figure 4 , the present invention provides a technical solution: a general chimeric antigen receptor regulatory T cell preparation method and application, comprising the following steps:
[0033] Isolation of S1, CD4+ regulatory T cells:
[0034] S101, selecting healthy persons to isolate mononuclear cells from peripheral blood or umbilical cord blood by gradient centrifugation;
[0035] S102. Using the magnetic bead method to isolate CD4+ T cells with a negative selection method, and then use a positive selection method to isolate CD4+CD25+ regulatory T cells;
[0036] S2. Preparation of lentivirus: use 293T cells to package the CAR expression system constructed by lentivirus, namely:
[0037] S201. After mixing the DNA of the lentiviral packaging system in proportion with a commercial transfection reagent, add the pre-cultured 293T cells together;
[0038] S202. After 48 hours, the cell supernatant is collected, and the supernatant is rich in lentiv...
Embodiment example 2
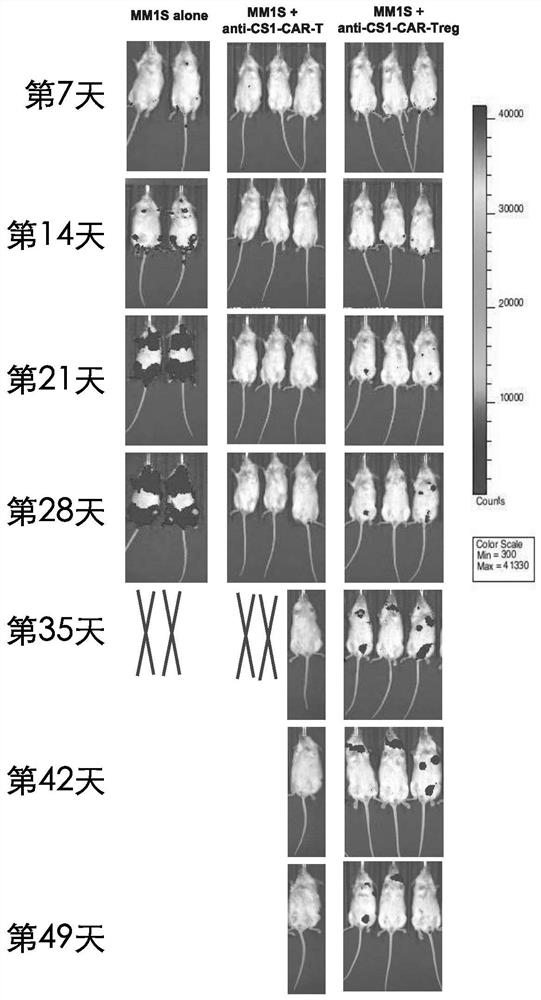
[0044] as attached Figure 1-3 As shown, a general chimeric antigen receptor regulatory T cell is applied in stem cell transplantation technology or tumor therapy.
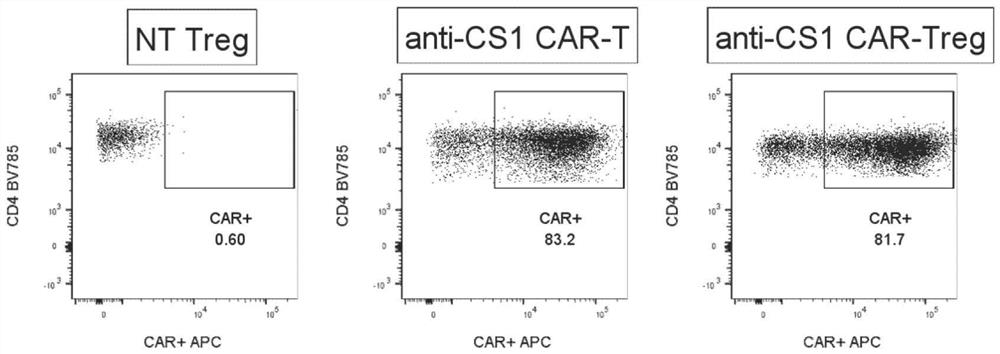
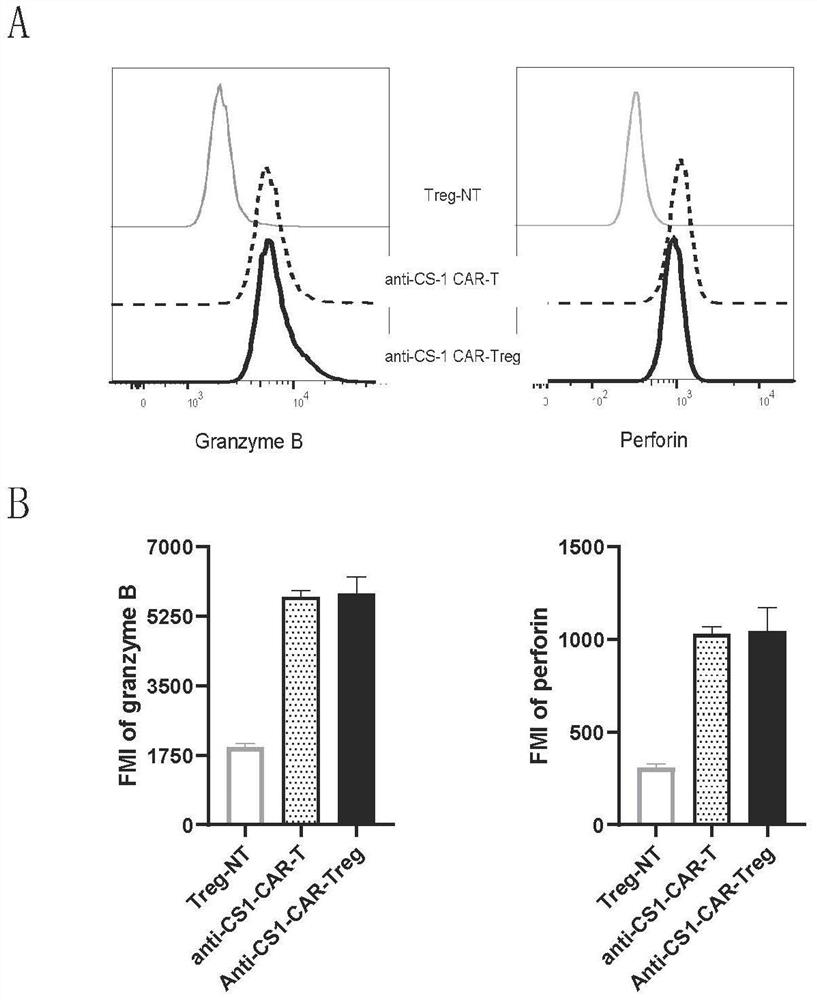
[0045] Taking CS1 as an example, CD4+ regulatory T cells in peripheral blood were transfected with a retrovirus carrying an anti-CS1 chimeric antigen receptor gene, so that CD4+ regulatory T cells expressed anti-cs1-CAR and generated anti-CS1 -CARTreg with greater than 90% transduction (see figure 1 ); afterward, anti-CS1-CARTreg was co-cultured with multiple myeloma cells (MM1s), and after 6 hours, anti-CS1-CARTreg was detected by flow cytometry for perforin and granzyme, and combined with attached figure 1 As shown, after co-culturing anti-CS1-CARTreg with multiple myeloma, CD4+Treg expressing CAR secreted a large amount of perforin and granzyme, while wild-type CD4+Treg (CD4+Treg not expressing CAR) did not Secretion of perforin and granzyme; this result indicates that the anti-CS1 chimeric antigen receptor o...
specific Embodiment example 1
[0050] Step 1. Purchase experimental materials:
[0051] Main instruments: biological safety cabinet (Haier, HR40-IIA2), CO 2 Incubator (Thermo, 3111), flow cytometer (BD, fortesaX20), cell counter (Nexcelom, CellometerAuto2000)
[0052] Reagents: FBS (Lonsera, S711-001S), X-vivo15 (Lonza, 04-418Q), Dynabeads CD3 / CD28 (Lifetechnology, 40203D), Ficoll (Dayou, DKW-LSH-0250), Luciferase detection (Promega, E6120) , anti-human CD3 (FITC, Biolegend, 300306), anti-human CD4 (BV785, BD, 740962), CAR-specific monoclonal antibody (self-standard), anti-human GranzymeB (PE / Dazzle594, Biolegend, 372216), anti-human Perforin ( BV421, Biolegend, 308122).
[0053] Step 2, the experimental method, is divided into the following five steps:
[0054] The first step, carrier construction
[0055] The NCBI website database searched the gene sequence information of human IgG1 hinge region, human CD28 transmembrane region, CD3z intracellular region and anti-CS-1 single chain antibody;
[0056] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com