Synthesis method of zolpidem hydrochloride
A technology for zolpidem hydrochloride and a synthesis method, which is applied in the field of synthesis of intermediate zolpidem hydrochloride, can solve the problems of low purity, insufficient reaction, low product yield and the like, and achieves wide adaptability and good effect, yield and purity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
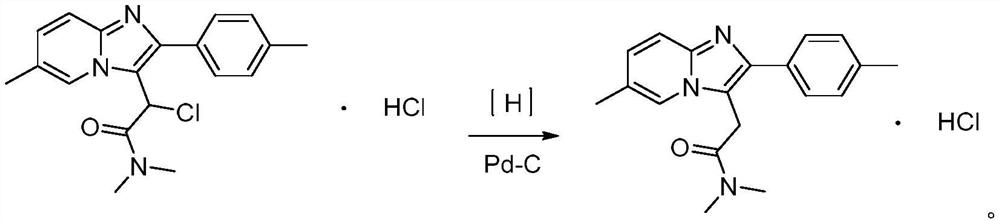
[0048] The present embodiment relates to a kind of preparation method of Zolpidem hydrochloride, comprises the following steps:
[0049] 1) 40g 2-chloro-N, N-dimethyl-2-(6-methyl-2-(p-tolyl) imidazo[1,2-a]pyridin-3-yl)acetamide hydrochloride Put the salt into the reactor, under the protection of nitrogen, add 200ml of methanol suspension containing 4.0g of Pd-C, pass in hydrogen, and react at 30-40°C for 2-3h;
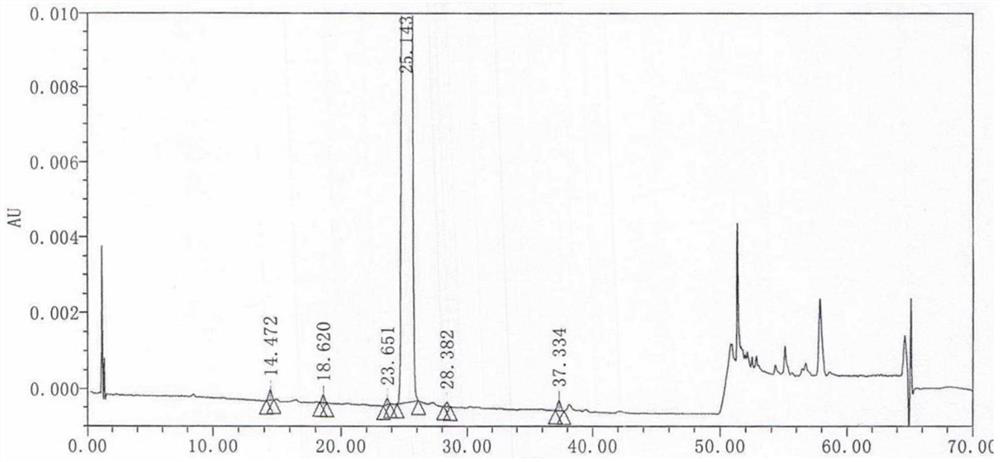
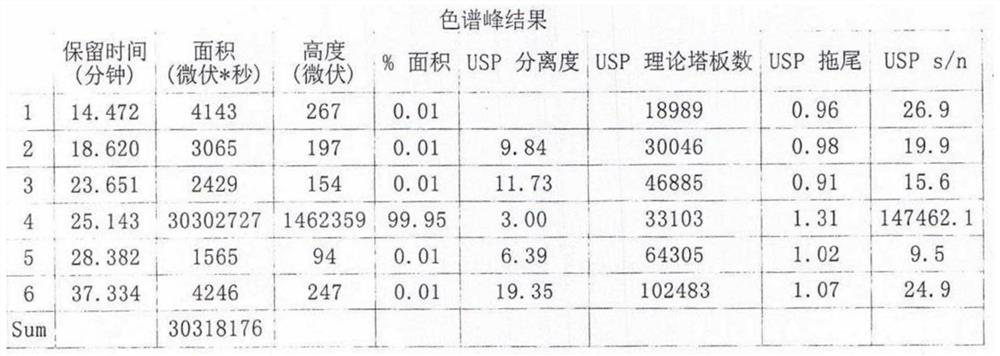
[0050] 2) Cool to below 30°C, filter, distill part of the methanol from the filtrate, control the temperature at 0~-5°C, freeze and crystallize for 8-20h, filter, dry for 2-4h, add 100ml of acetone to reflux for 20-30min, cool down 0~- Cool and crystallize at 5°C for 8-20h. Filter and wash with a small amount of acetone to obtain white solid crystals, dry at 40-60°C for 4 hours to obtain 26.57g of Zolpidem hydrochloride, the yield is 73.07%, the purity is 99.95%, ESI: m / z [M+H ] + 344.18. The resulting product was analyzed by liquid chromatography, and its chromato...
Embodiment 2
[0052] This embodiment relates to a preparation method of Zolpidem hydrochloride, compared with Example 1, the difference is:
[0053] Amplify 2-chloro-N,N-dimethyl-2-(6-methyl-2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)acetamide hydrochloride to 1.0Kg, the yield is 78.93%, and the purity is 98.39%.
Embodiment 3
[0055] This example relates to a preparation method of Zolpidem hydrochloride. Compared with Example 1, the difference is that methanol is replaced by isopropanol, the yield is 72.35%, and the purity is 95.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com