Method for preparing rare earth fluoride by using fluorinated ionic liquid
A technology of rare earth fluoride and fluoride ions, which is applied in the preparation/treatment of rare earth metal compounds, rare earth metal halides, rare earth metal fluorides, etc., can solve the problems of energy consumption environment, fluorine gas generation, disadvantages, etc., and achieve reaction Effects of mild conditions and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
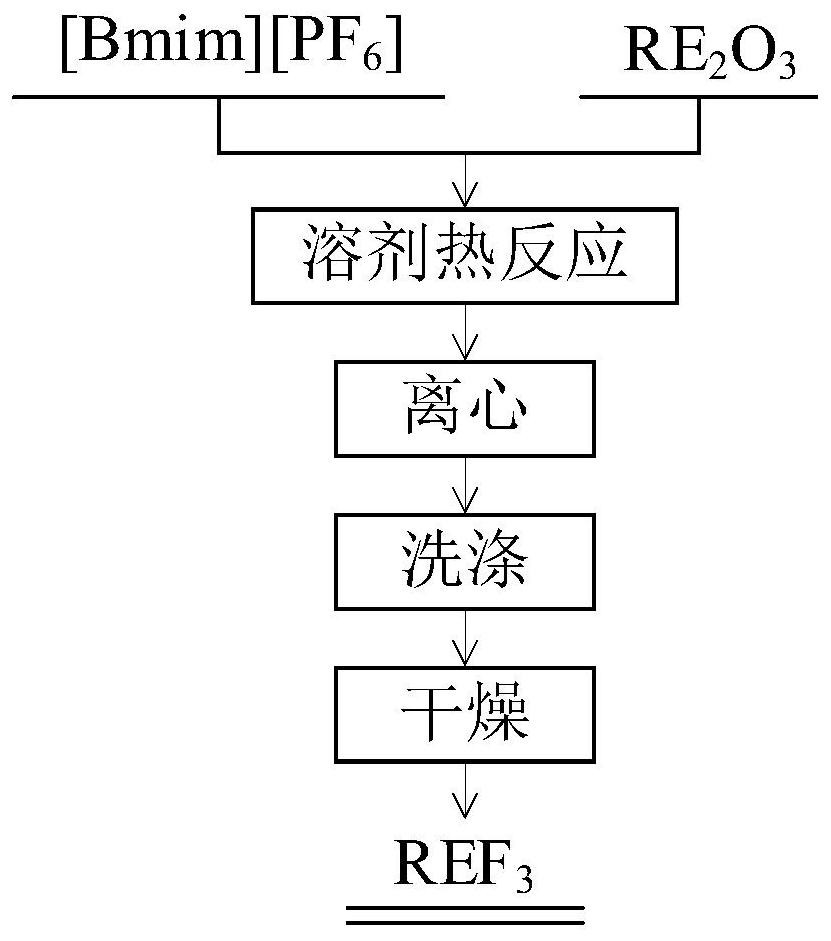
[0032] Refer figure 1 Using fluorinated ionic liquid [Bmim] [PF 6 ] Direct preparation of rare earth fluoride NdF 3 The method comprising the steps of:
[0033] (1) Weigh 1.12mmol Nd 2 O 3 6mL solid powder and fluorinated ionic liquid [Bmim] [PF 6 ] In 20mL Teflon liner, after a Teflon-lined stainless steel reaction kettle placed;
[0034] (2) A stainless steel autoclave, an oven, heat the reaction solvent, the reaction temperature was controlled 180 ℃, reaction time 48h, the use of ionic liquids will heat the solid ion Nd 2 O 3 Into a solid at a high temperature and pressure NdF 3 ; Until completion of the reaction, the reaction product was removed by centrifugation, 8000 rpm for controlling the rotational speed centrifugation, the centrifugation time 5min, solid-liquid separation;
[0035] (3) The precipitate was washed three times with anhydrous ethanol, to remove residual ionic liquid;
[0036] (4) After completion of washing the precipitate was dried at 80 ℃ for 12h, to obtai...
Embodiment 2
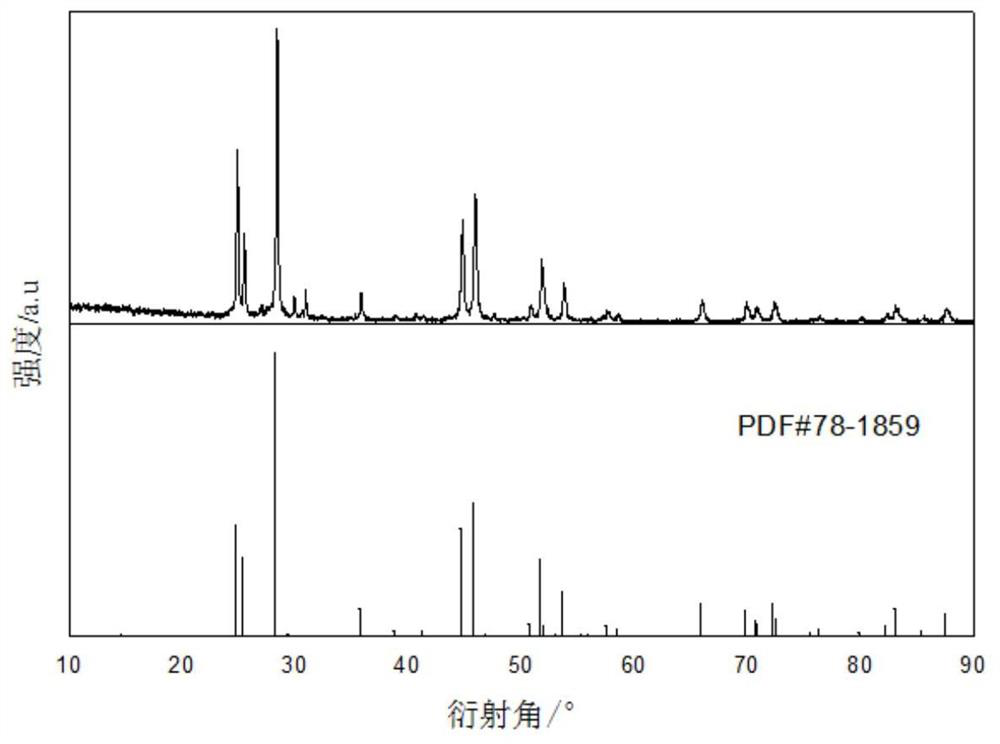
[0039] Using fluorinated ionic liquid [BMIM] [PF 6 ] Directly prepare fluorinated rare earth LAF 3 Method, including the steps of:
[0040] (1) Weigh 0.56mmol LA 2 O 3 Solid powder and 6 mL fluorinated ionic liquid [BMIM] [PF 6 The polytetrafluoroethylene liner is placed in a stainless steel reaction kettle in a 20 ml polytetrafluorine liner.
[0041] (2) Put the stainless steel reactor into the oven, heat reaction, the temperature of the reaction temperature is 160 ° C, the reaction time is 96h, and the solid La 2 O 3 Convert to solid LAF under high temperature high pressure 3 At the end of the reaction, the reaction product was centrifuged, and the centrifugation speed was 6000 rpm, centrifugation time was 10 min, and solid-liquid separation was achieved.
[0042] (3) Wash the precipitate three times with anhydrous ethanol, remove the residual ionic liquid;
[0043] (4) After the washing is completed, the precipitate is dried under 80 ° C for 18 h, which can get LAF. 3 Powder.
...
Embodiment 3
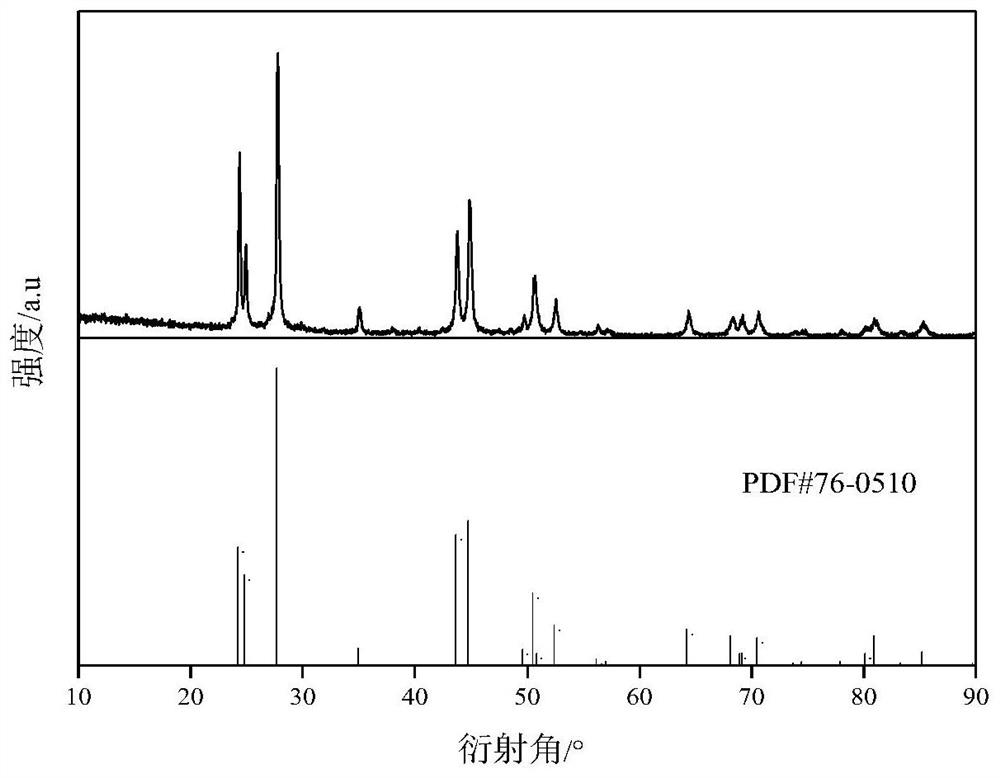
[0046] Using fluorinated ionic liquid [BMIM] [PF 6 ] Directly prepare fluorinated rare earth CEF 3 Method, including the steps of:
[0047] (1) Weigh 1.12mmol CE 2 O 3 Solid powder and 9 ml of fluorinated ionic liquid [BMIM] [PF 6 The polytetrafluoroethylene liner is placed in a stainless steel reaction kettle in a 20 ml polytetrafluorine liner.
[0048] (2) Put the stainless steel reactor into the oven, heat reaction, the temperature of the reaction temperature is 200 ° C, the reaction time is 24 h, and the solid CE 2 O 3 Convert to solid CEF under high temperature high pressure 3 At the end of the reaction, the reaction product was centrifuged, and the centrifugation speed was 10000 rpm, centrifugation time was 3 min, and solid-liquid separation was achieved.
[0049] (3) Wash the precipitate 4 times with anhydrous ethanol, remove the residual ionic liquid;
[0050] (4) After the washing is completed, the precipitate is dried under 90 ° C for 12 h, which can be obtained from CEF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com