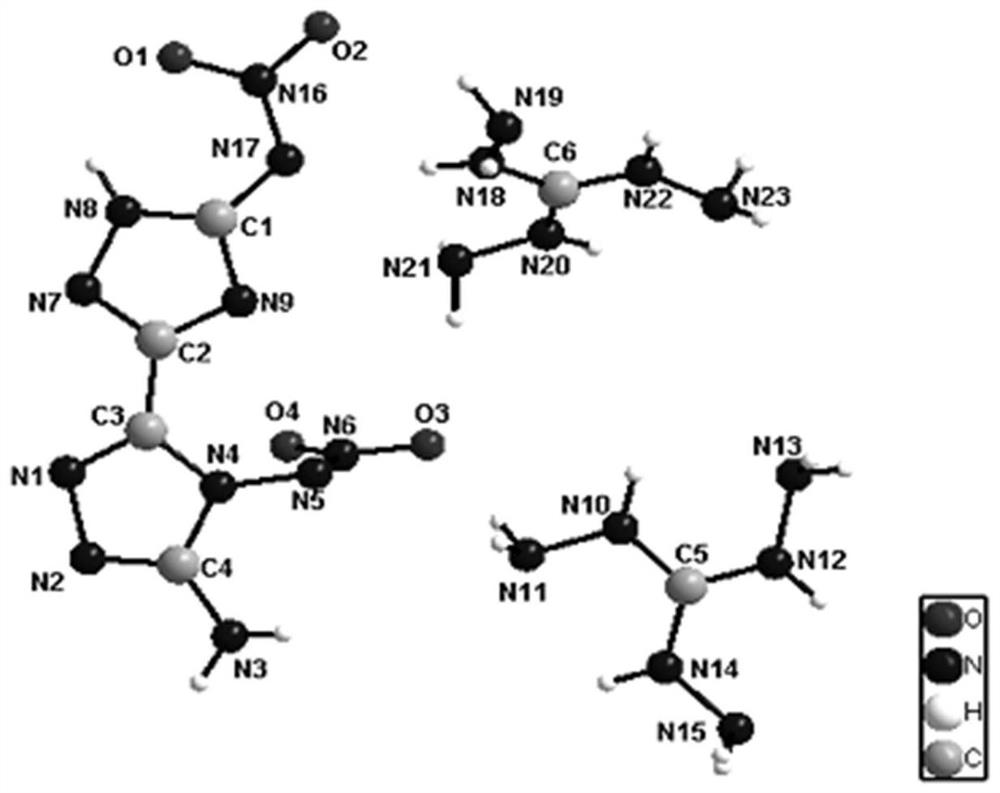
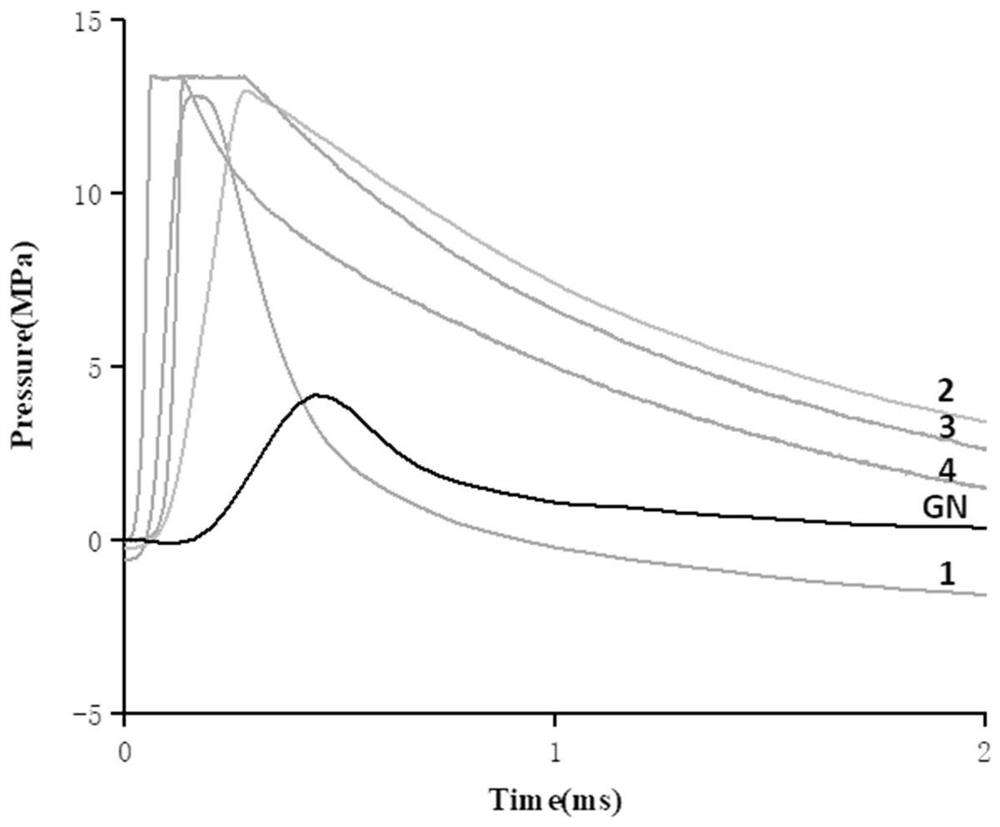
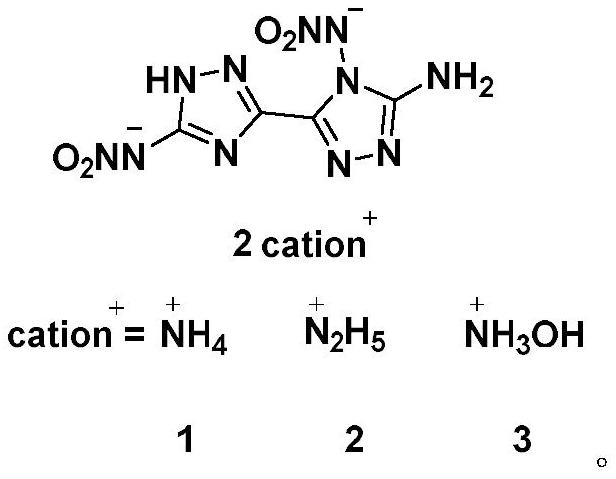
4, 5'-dinitramino-5-amino-3, 3'-bis-1, 2, 4-triazole energetic ionic salt and synthesis method thereof
The technology of a dinitroamine group and a synthesis method, which is applied in the field of gas generating agents, can solve problems such as unfavorable environment treatment, hidden safety hazards, restricted use, etc., and achieves the effect of good gas production effect and no residue remaining.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: ring closing reaction
[0046]
[0047] Add 12.8 g of 2-amino-5-carboxy-1,2,4-triazole (100 mmol) and 15 g of 1,3- Diaminoguanidine hydrochloride (120 mmol), heated up to 120° C., reacted for 4 hours, and the reaction liquid was clear. Quench the reaction solution with 200mL of ice water, adjust the pH of the reaction solution to 7~8 with concentrated sodium hydroxide solution, a large amount of solids precipitate out of the reaction system, and filter to obtain 4,5,5'-triamino-3,3 '-1,2,4-triazole, the yield was 3 g (yield: 16.5%).
[0048] 1 H NMR (d 6 -DMSO, 25°C): δ=12.4, 6.15, 5.80, 5.71ppm. 13 C NMR(d 6 -DMSO, 25°C): δ=157.48, 154.25, 148.84, 142.12ppm. IR (KBr): v=3735,3416,3159,2624,1627,1557,1473,1368,1309,1257,1126,1051,983,889,752,712 cm -1 .
Embodiment 2
[0049] Embodiment 2: Nitrification reaction
[0050]
[0051] 1.5 g of 4,5,5'-triamino-3,3'-1,2,4-triazole (8.28 mmol) was slowly added to 10 mL of 100% nitric acid solution, and reacted at -15°C for 20 hours. 100mL of ice water quenched the reaction solution, and a large amount of solids were precipitated. After filtration, 4,5'-dinitroamino-5-amino-3,3'-linked-1,2,4-triazole was obtained. The yield was 1 g (yield: 70%).
[0052] 1 H NMR (d 6 -DMSO, 25°C): δ=8.45, 5.12ppm. 13 C NMR(d 6 -DMSO, 25°C): δ=152.73, 149.96, 140.56, 139.36ppm.IR (KBr): v=3589,3392,3231,3144,2707,1694,1644,1584,1500,1406,1336,1265,1221 ,1093,996,958,878,840,776,731cm -1 .
Embodiment 3
[0053] Embodiment 3: Salt-forming reaction
[0054]
[0055] Add alkaline solution (ammonia water, hydrazine hydrate , hydroxylamine aqueous solution) until the reaction solution was clear, and the reaction system was neutral at this time, and reacted at 60° C. for 3 hours. Stand still and cool down to precipitate a precipitate, and filter to obtain the relevant energetic ion salt (1-3). 4,5'-Dinitroamino-5-amino-3,3'-bi-1,2,4-triazole ammonium salt (1):
[0056] 1 H NMR (d 6 -DMSO, 25°C): δ=7.41, 5.91ppm. 13 C NMR(d 6 -DMSO, 25℃): δ=157.16, 153.31, 149.23, 141.91ppm. IR (KBr): v=3044,1682,1621,1560,1524,1383,1222,1138,1083,1041,974,882,765,712,674,624cm -1 .
[0057] 4,5'-Dinitroamino-5-amino-3,3'-bi-1,2,4-triazole hydrazine salt (2):
[0058] 1 H NMR (d 6 -DMSO, 25°C): δ=8.05, 6.29ppm. 13 C NMR(d 6 -DMSO, 25°C): δ=157.36, 153.13, 148.62, 141.75ppm. IR (KBr): v=3149,2700,1500,1434,1343,1260,1121,1083,1008,970,866,787,762,730,669cm -1 .
[0059] 4,5'-Dinitroam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com