A method for synthesis of organic iodides, a perovskite-forming composition comprising an organic iodide and a photovoltaic cell with a perovskite layer obtained therefrom
A technology of hydrogen iodide and organic cations, applied in the preparation of lead organic compounds, organic compound/hydride/coordination complex catalysts, organic compounds, etc., can solve the problem of crystallization reaction rate reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
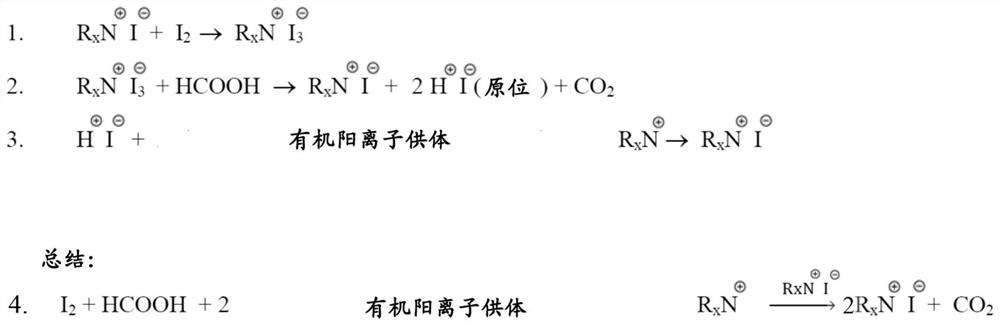
[0065] The developed preparation R x The process of NI compounds ensures improved purity of the resulting products, which in turn enables them to be used as perovskite precursors in various perovskite-forming compositions, including colloidal solutions, such as inks, for the production of Morphology and quality of perovskite crystals in perovskite coatings.
[0066] The developed iodide synthesis method differs from prior art methods in that the method according to the present disclosure involves The step of obtaining hydrogen iodide (HI) in situ in the reaction medium . In other words, according to the developed method, hydrogen iodide (HI) was not used as a direct substrate for the synthesis of the respective iodides. Instead, the developed method includes the step of supplying substrates suitable for in situ hydrogen iodide (HI) synthesis into the reaction medium.
[0067] The developed method allows to obtain as having the molecular formula R x Various compounds of th...
Embodiment I
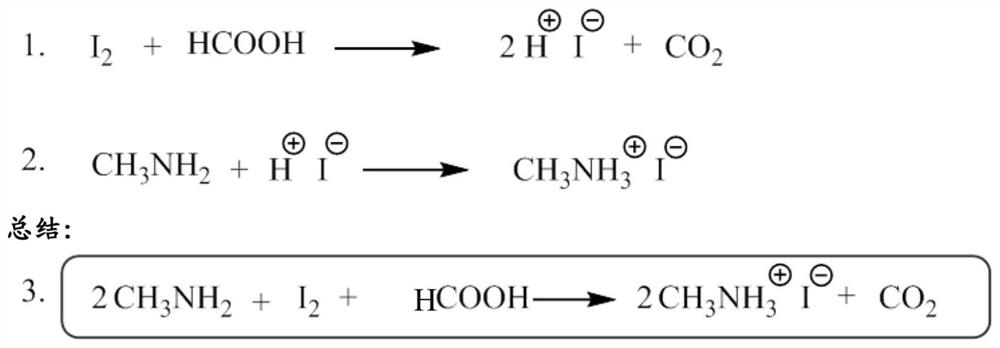
[0117] Example 1 - Synthesis of methylammonium iodide (MAI) using MAI as catalyst:
[0118] In a round bottom flask, dissolve a portion of the MAI catalyst in ethanol for each I 2 Iodine was added to MAI in an amount of 0.01 molar to obtain a bright translucent solution. Then, the whole dark brown I 2 Iodine was added to the resulting solution in one step to obtain a brown, opaque solution, which confirmed the presence of iodine (I 2 ). After 5 minutes, it dissolved, indicating that I was formed in solution 3 - (otherwise I 2 Molecular iodine will dissolve at a much slower rate). Formic acid is then added in a single step relative to I 2 Iodine was added to the resulting mixture in a molar ratio of 0.97:1.0, macroscopically, no change was observed in the flask. A suitable organic cation donor, methylamine, is then added to the solution. Methylamine was slowly added dropwise to the solution so that every mole of I added to the solution 2 Iodine has a total of 2.6 mo...
Embodiment II
[0120] Example II - Synthesis of catalyst-free octyl ammonium iodide (OAI):
[0121] Part of the iodine (I 2 ) was dissolved in ethanol in a round bottom flask in a single step to obtain a non-translucent dark brown solution demonstrating that molecular iodine (I 2 )The presence. After 15 minutes, iodine dissolved in ethanol and the solution remained dark brown and opaque. Then with 0.97:1.0 HCOOH:I 2 Molar ratios of formic acid were added to the flask and no change was observed in the flask. Then 2.6 moles of octylamine / per mole of I 2Iodine was slowly added dropwise to the solution. After addition of each drop of octylamine, bubbles and foam appeared in the reaction solution and an increase in temperature of the reaction mixture was observed due to its exothermic process. Bubbles and foam indicate the reaction of octylamine with in situ generated hydrogen iodide HI. After all the octylamine had been added (the reaction mixture remained brown), the flask was heated f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com