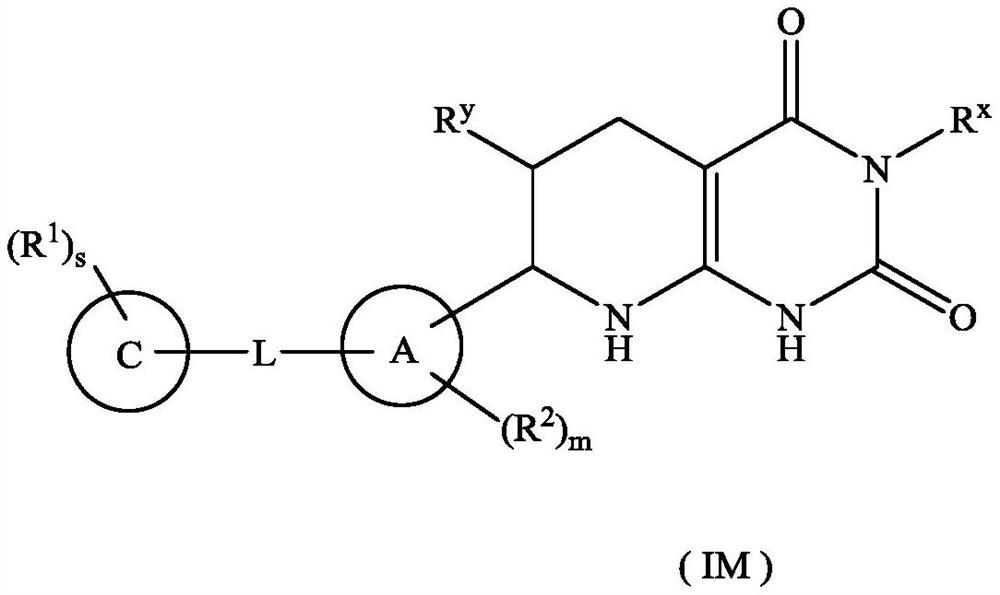
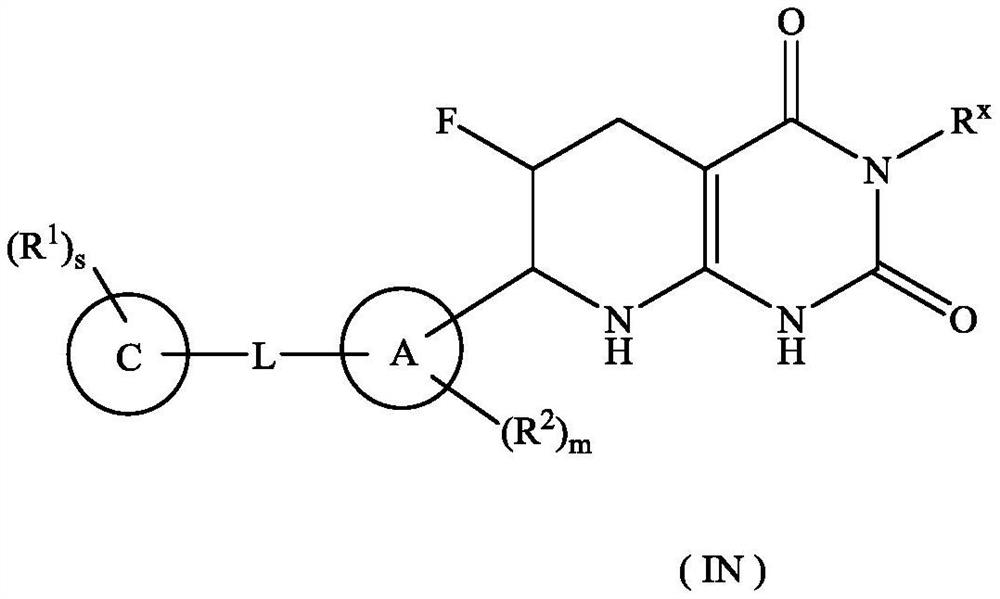
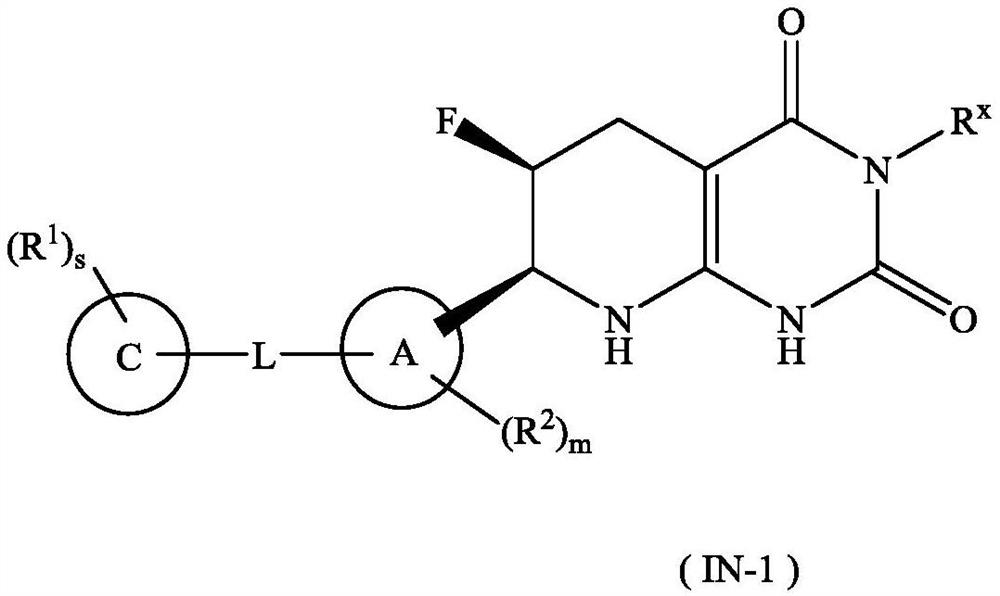
Tetrahydropyridopyrimidinedione derivative, preparation method thereof and application of tetrahydropyridopyrimidinedione derivative in medicine
A compound and mixture technology, applied in the field of medicine, can solve problems such as treating the symptoms but not the root cause
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0514] (6S,7S)-7-(5-cyclopropyl-2-fluorophenyl)-6-fluoro-3-(tetrahydro-2H-pyran-4-yl)-5,6,7,8- Tetrahydropyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione 1
[0515]
[0516] first step
[0517] (2R,3S)-3-(5-Bromo-2-fluorophenyl)-3-((R)-1,1-dimethylethylsulfinamido)-2-fluoropropionic acid ethyl ester 1c
[0518] (R,E)-N-(5-bromo-2-fluorobenzylidene)-2-methylpropane-2-sulfinamide 1a (8.6g, 28.2mmol, using the literature "J.Med.Chem , 2019,62,9618-9641" prepared by known method), ethyl 2-fluoroacetate 1b (4.5g, 42.3mmol, Shanghai Titan Technology Co., Ltd.) and N,N,N',N'-tetramethyl Ethylenediamine (6.6 g, 56.4 mmol, Shanghai Aladdin Biochemical Technology Co., Ltd.) was dissolved in anhydrous THF (90 mL). After cooling to -70°C, a 1M solution of lithium bis(trimethylsilyl)amide in tetrahydrofuran (42.3 mL, 42.3 mmol, Shanghai Titan Technology Co., Ltd.) was added dropwise. Under nitrogen protection, the reaction was stirred at -70°C for 3 hours. The reaction was quenched by addi...
Embodiment 2
[0546] (6S,7S)-6-fluoro-7-(2-fluoro-5-((6-(trifluoromethyl)pyridin-3-yl)oxy)phenyl)-3-(tetrahydro-2H- Pyran-4-yl)-5,6,7,8-tetrahydropyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione 2
[0547]
[0548] first step
[0549] 2-Fluoro-5-((6-(trifluoromethyl)pyridin-3-yl)oxy)benzaldehyde 2c
[0550] 2-Fluoro-5-hydroxybenzaldehyde 2a (500 mg, 3.57 mmol, adamas) and 5-fluoro-2-(trifluoromethyl)pyridine 2b (1.2 g, 7.27 mmol, adamas) were dissolved in N,N-di Add methylformamide (10 mL), potassium carbonate (1.0 g, 7.25 mmol), and react at 110°C for 0.5 hours. Add water (50 mL), extract with ethyl acetate (20 mL×2), combine the organic phases, concentrate under reduced pressure, and purify the resulting residue by silica gel column chromatography with eluent system A to obtain the title product 2c (300 mg, yield : 29.5%).
[0551] MS m / z (ESI): 285.8 [M+1].
[0552] second step
[0553] (R)-N-(2-fluoro-5-((6-(trifluoromethyl)pyridin-3-yl)oxy)benzylidene)-2-methylpropane-2-sulfinamide 2e...
Embodiment 3
[0581] (6S,7S)-6-fluoro-7-(2-fluoro-5-((6-methylpyridin-3-yl)oxy)phenyl)-3-(tetrahydro-2H-pyran-4 -yl)-5,6,7,8-tetrahydropyrido[2,3-d]pyrimidine-2,4(1H,3H)-dione 3
[0582]
[0583]
[0584] first step
[0585] 2-Fluoro-5-((6-methylpyridin-3-yl)oxy)benzaldehyde 3c
[0586] 6-Methylpyridin-3-ol 3a (1.0g, 9.16mmol, Shanghai Bide Pharmaceutical Technology Co., Ltd.) and (4-fluoro-3-formylphenyl) boronic acid 3b (2.0g, 11.9mmol, Shanghai Han Hong Chemical Technology Co., Ltd.) was dissolved in dichloromethane (10mL), added pyridine (1.5g, 18.8mmol, adamas), triethylamine (1.9g, 18.8mmol, adamas), anhydrous copper acetate (3.4g, 18.8 mmol, Shanghai Bi De Pharmaceutical Technology Co., Ltd.), reacted at room temperature for 24 hours. After filtration and concentration under reduced pressure, the resulting residue was purified by silica gel column chromatography with eluent system A to obtain the title product 3c (650 mg, yield: 30.7%).
[0587] MS m / z (ESI): 231.9 [M+1]. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com