Construction and application of recombinant protein based on lactobacillus LPxTG motif
A technology of recombinant protein and lactic acid bacteria, applied in the field of lactic acid bacteria, to achieve the effect of good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
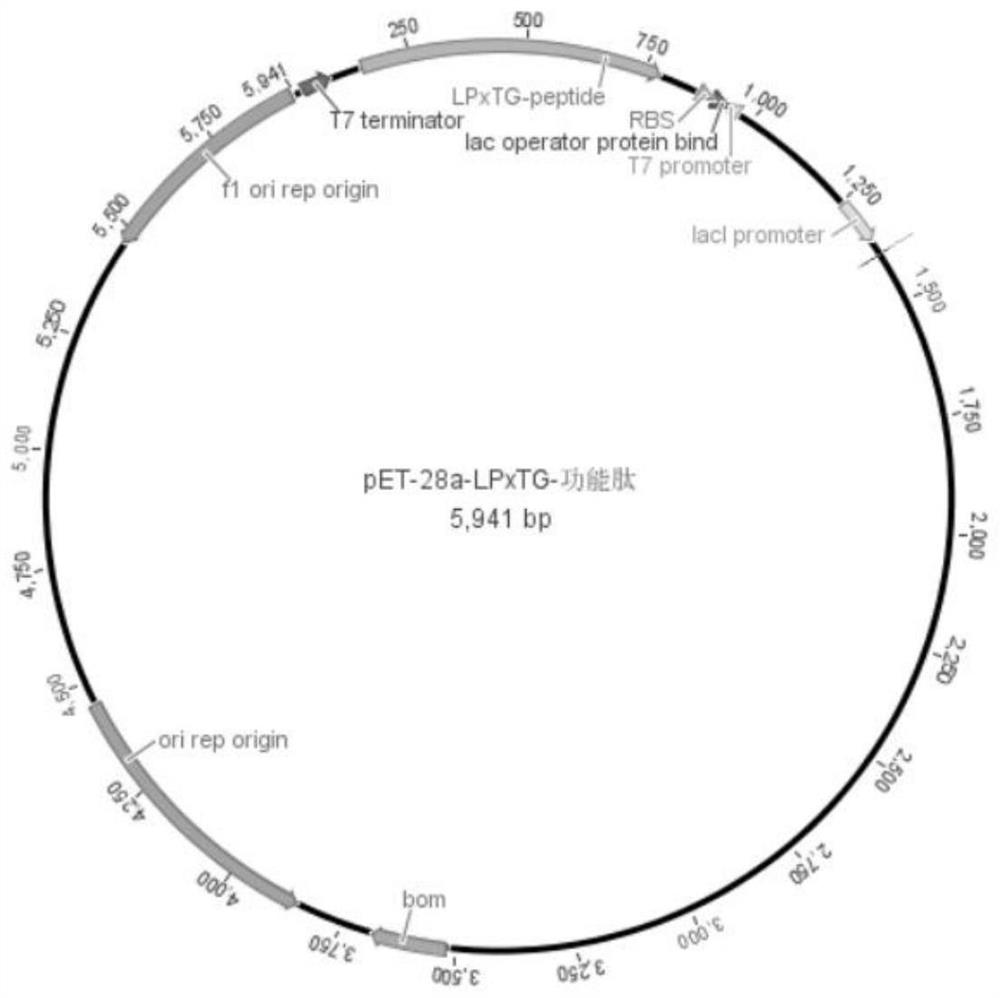
[0053] Embodiment 1: Construction method based on lactic acid bacteria LPxTG motif recombinant protein
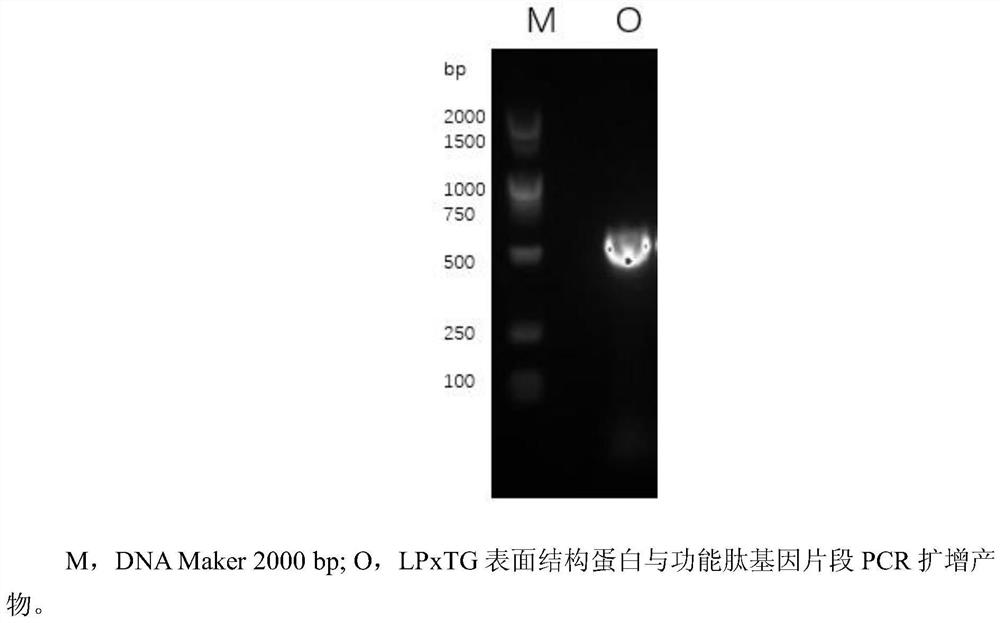
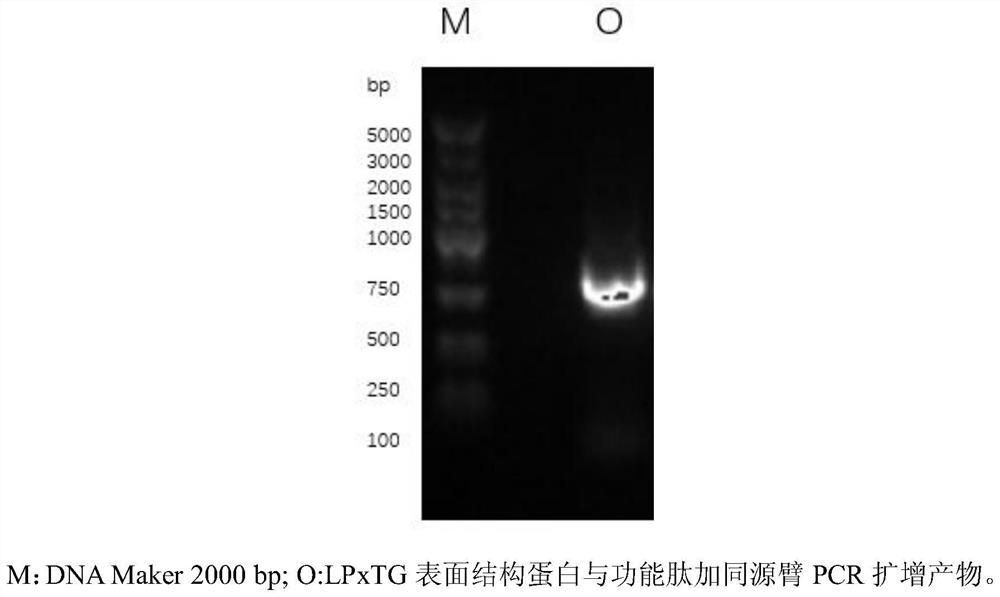
[0054] 1.1 obtain the gene fragment of LPxTG-functional peptide (step S1)
[0055] According to the gene sequence of the surface structural protein containing the LPxTG sequence in Lactobacillus reuteri published on NCBI:
[0056] (As shown in SEQ ID NO.1), use CE Design to design primers to obtain primers F1 and R1, and increase the sequence of functional peptides in primer F1 (in this embodiment, functional peptides are antioxidant peptides, and its amino acid sequence is shown in the table 1. After the functional peptide was synthesized by Shanghai Sangon Biological Co., Ltd., corresponding to the F1' of the primer, see Table 1, and the designed primer sequence was sent to Shanghai Sangon Biological Co., Ltd. for synthesis. The whole genome of Lactobacillus reuteri SH23 was extracted with the Easy Pure BacteriaGenomic DNA Kit, and the DNA concentration was verified with...
Embodiment 2
[0070] Example 2: Comparative study on the functional properties of LPxTG-functional peptides and functional peptides
[0071] In Example 2, in order to reflect the antioxidant capacity of the functional peptide, the functional peptide was named as an antioxidant peptide. That is, the LPxTG-functional peptide in Example 1 is the LPxTG-antioxidative peptide in Example 2; the functional peptide in Example 1 is the antioxidant peptide in Example 2.
[0072] In order to compare the scavenging activities of LPxTG-antioxidant peptides and antioxidant peptides on hydroxyl radicals, ABTS radicals and DPPH radicals, the following experiments were carried out.
[0073] 2.1 Hydroxyl radical scavenging activity
[0074] Divided into 8 groups. Among them, group 1 has only antioxidant peptide (i.e. peptide), the concentration is 5 μg / ml, that is, dissolving 5 μg antioxidant peptide per milliliter of distilled water; group 2 is only LPxTG-antioxidant peptide (i.e. LPxTG-peptide), the conce...
Embodiment 3
[0088] Example 3: Test method for gastrointestinal tolerance of LPxTG-functional peptides
[0089] In Example 3, in order to reflect the antioxidant capacity of the functional peptide, the functional peptide was named antioxidant peptide. That is, the LPxTG-functional peptide in Example 1 is the LPxTG-antioxidative peptide in Example 3; the functional peptide in Example 1 is the antioxidant peptide in Example 3.
[0090] In order to test the gastrointestinal tolerability properties of LPxTG-antioxidant peptides, the following experiments were performed. Artificial gastric juice (hereinafter referred to as gastric juice for short) and artificial intestinal juice (hereinafter referred to as intestinal juice for short) were prepared respectively. The preparation method of artificial gastric juice is to add 0.5g NaCl, 0.3g pepsin and dilute to 100ML with ultrapure water, pH=2. The preparation method of artificial intestinal juice is to add 0.5g NaCl, 0.5g ox bile salt, 0.3g trypsi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com