Narrow-band gap polymer based on benzobithiadiazole or thiadiazole quinoxaline as well as preparation method and application of narrow-band gap polymer
A technology of thiadiazole quinoxaline and benzobisthiadiazole is applied in the field of narrow-bandgap polymers and their preparation, which can solve the problems of high toxicity of organic tin salts, complicated synthesis steps and the like, and achieves few synthesis steps and high electron density. Mobility, simple preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] This embodiment provides a narrow bandgap polymer, its structural formula is:
[0061]
[0062] Among them, R 2 For 2-octyl dodecyl.
[0063] The preparation method and reaction equation of this polymer are as follows:
[0064]
[0065] Trisdibenzylideneacetone dipalladium (Pd 2 (dba) 3 ) is the catalyst, and three p-benzyloxyphosphorus ((o-MeOPh) 3 P) is the ligand of the above catalyst, potassium carbonate (K 2 CO 3 ) plays the role of neutralizing the acid produced by the reaction and activating the catalyst, trimethylacetic acid (PivOH) acts as a catalytic proton shuttle, cooperates with the palladium center to reduce the cracking energy of the C-H bond, and promotes the reaction. 0.097mmol compound 2, 0.097mmol compound 5, 0.005mmol tridibenzylidene acetone dipalladium (Pd 2 (dba) 3 ), 0.01mmol tri-p-phenylmethoxyphosphorus ((o-MeOPh) 3 P), 0.05mmol trimethylacetic acid (PivOH) and 0.5mmol potassium carbonate (K 2 CO 3 ) was dissolved in 0.5 mL o-x...
Embodiment 2
[0068] This embodiment provides a narrow bandgap polymer, its structural formula is:
[0069]
[0070] Among them, R 2 For 2-octyl dodecyl.
[0071] The preparation method and reaction equation of this polymer are as follows:
[0072]
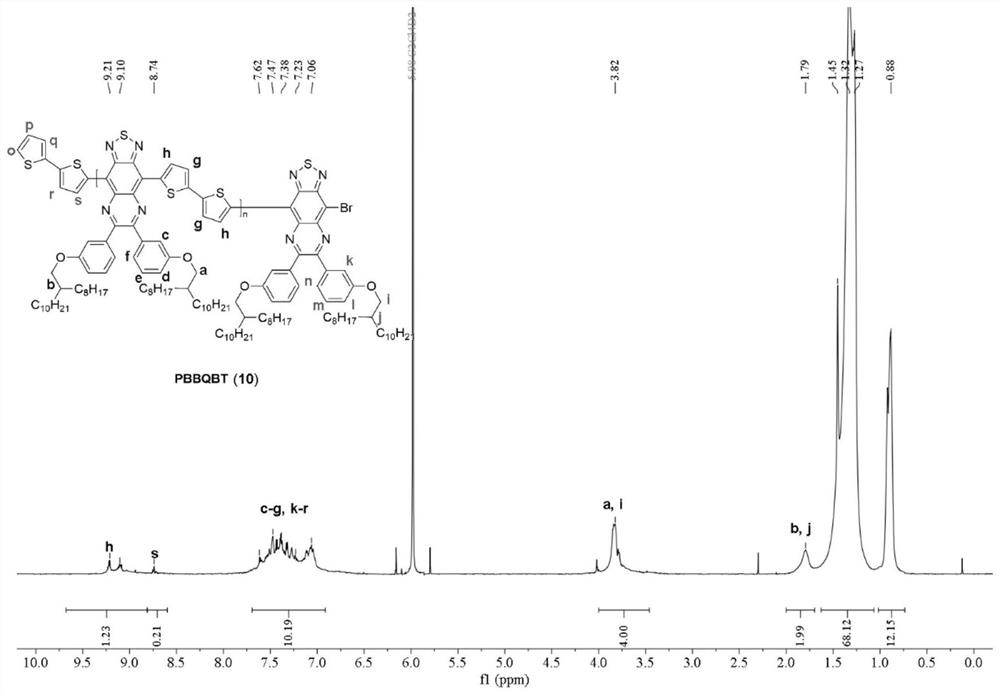
[0073] The mechanism, process and aftertreatment of this reaction are the same as in Example 1. The dosage is: 0.092mmol compound 2, 0.092mmol compound 6, 0.005mmol tridibenzylidene acetone dipalladium (Pd 2 (dba) 3 ), 0.01mmol tri-p-phenylmethoxyphosphorus ((o-MeOPh) 3 P), 0.05mmol trimethylacetic acid (PivOH) and 0.5mmol potassium carbonate (K 2 CO 3 ) was dissolved in 0.46mL o-xylene. The final post-treatment gave the above-mentioned polymer, denoted as polymer 11, with a yield of 92%.
[0074] figure 2 For the proton nuclear magnetic spectrum (500MHz, C of the polymer 11 that embodiment 2 obtains 2 D. 2 Cl 4 , 100°C).
Embodiment 3
[0076] This embodiment provides a narrow bandgap polymer, its structural formula is:
[0077]
[0078] Among them, R 2 is 2-octyldodecyl, R 6 For dodecyl.
[0079] The preparation method and reaction equation of this polymer are as follows:
[0080]
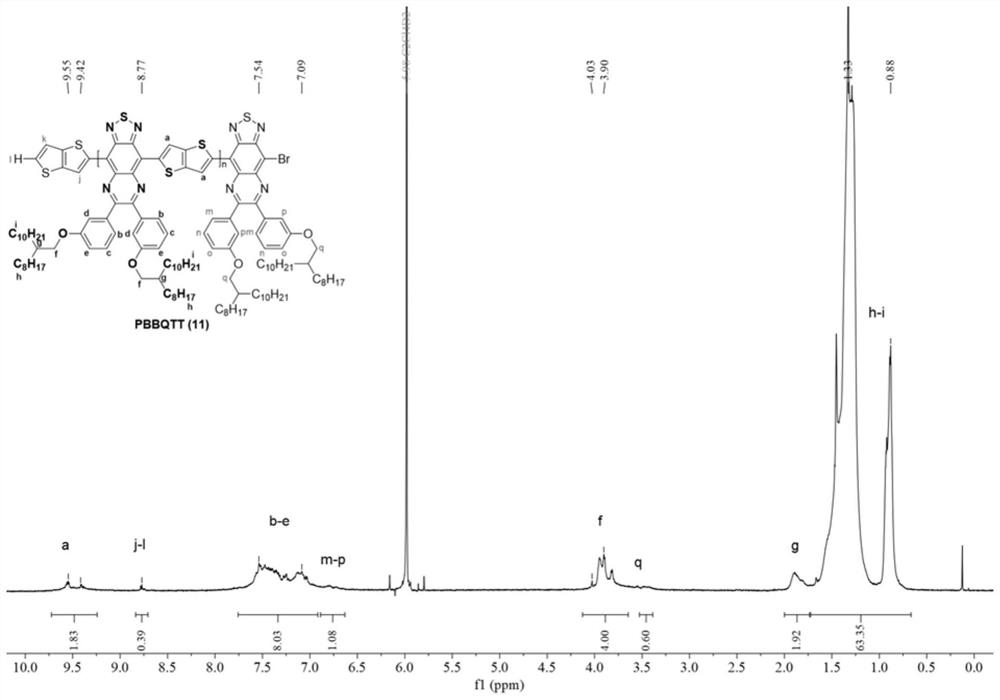
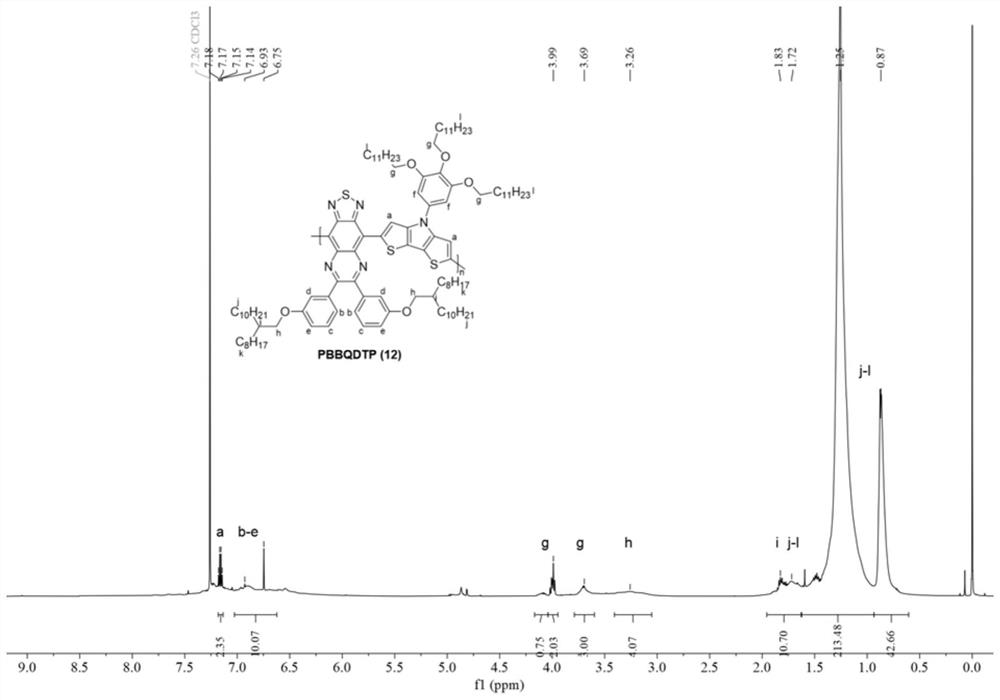
[0081] The mechanism, process and aftertreatment of this reaction are the same as in Example 1. The dosage is: 0.092mmol compound 2, 0.092mmol compound 7, 0.005mmol tridibenzylidene acetone dipalladium (Pd 2 (dba) 3 ), 0.01mmol tri-p-phenylmethoxyphosphorus ((o-MeOPh) 3 P), 0.05mmol trimethylacetic acid (PivOH) and 0.5mmol potassium carbonate (K 2 CO 3 ) was dissolved in 0.46mL o-xylene. The final post-treatment gave the above-mentioned polymer, denoted as polymer 12, with a yield of 38%.
[0082] image 3 For the proton nuclear magnetic spectrum (500MHz, C of the polymer 12 that embodiment 3 obtains 2 D. 2 Cl 4 , 100°C). Polymer 12 can confirm that the two monomers have been copolymerized through NMR spectra ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com