Preparation method of 2, 6-dihalogenated methyl benzoate
A technology of methyl dihalogenated benzoate and dihalogenated benzoic acid is applied in the field of preparation of methyl 2,6-dihalogenated benzoate, and can solve the problem that it is difficult to realize large-scale production and application and the environmental pollution of the generation process. , the complex reaction device and other problems, to achieve the effect of easy reaction operation and purification process, high reaction yield and simple reaction device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
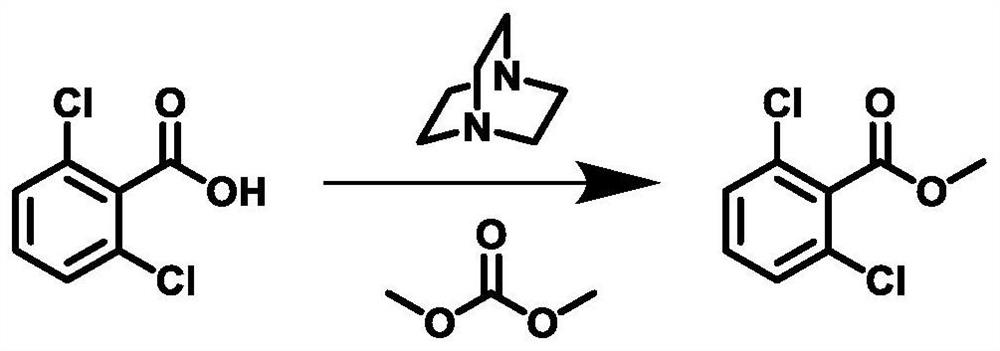
[0036] Preparation method of 2,6-dichlorobenzoate methyl ester, specific synthetic route figure 1 Indicated.
[0037] 1) 2,6-dichlorobenzoic acid (38.2 g, 200 mmol), dimethyl carbonate (305.6 g), and triamiacarbonate (35.6 g) and triamiacarbonate ( Dabco, 22.4 g, 200 mmol, stirred at room temperature. The temperature was warmed to 90 ° C, and the insulation reaction was 10 h.
[0038] 2) After the reaction is completed, stop heating, and the reaction is cooled to room temperature. The solvent was evaporated under reduced pressure, and 152.8 g of water was added to the residual liquid, stirred vigorously, extracted with ethyl acetate. Ethyl acetate layer is sequentially with water, dilute hydrochloric acid (5%), saturated sodium hydrogencarbonate, and water, the organic phase is dried and concentrated, that is, 2,6-dichlorobenzoate, weight of about 40g. 97.5% yield, the content is 98.680%, and the results are Figure 4 Indicated. 1 H NMR (400MHz, CDCL 3 : Δ7.33-7.20 (m, 3H), 3.97 (S...
Embodiment 2
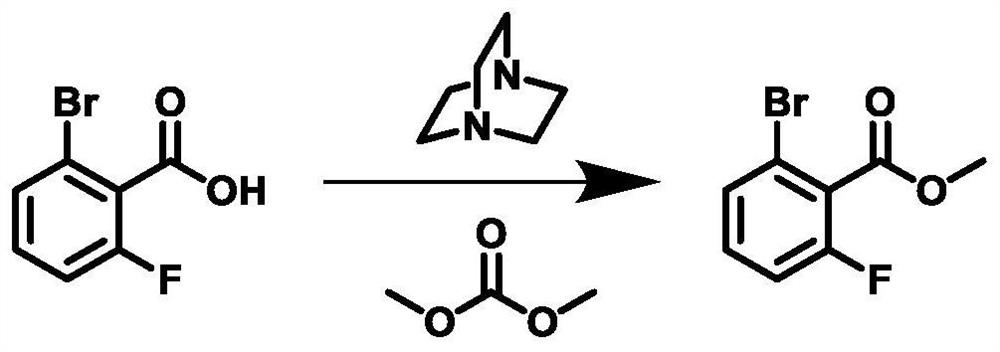
[0040] Method for preparing 2-bromo-6-fluorobenzoate methyl ester, specific synthetic route figure 2 Indicated.
[0041] 1) 2-bromo-6-fluorobenzoic acid (43.8 g, 200 mmol), dimethyl carbonate (305.6 g) and DABCO (22.4 g) were sequentially added to the 1L tetra-necked flask equipped with a stirrer, reflow tube, and thermometer. , 200 mmol), stirred at room temperature. The temperature was warmed to 90 ° C, and the insulation reaction was 10 h.
[0042] 2) After the reaction is completed, stop heating, and the reaction is cooled to room temperature. The solvent was evaporated under reduced pressure, and 152.8 g of water was added to the residual liquid, stirred vigorously, extracted with ethyl acetate. Ethyl acetate layer is sequentially with water, dilute hydrochloric acid (5%), saturated sodium hydrogencarbonate, and water, the organic phase is dried, and then the pale yellow oily liquid, i.e., 2-bromo-6-fluorobenzoate, weighs about 45g. 96.5% yield, the content is 98.257%, the re...
Embodiment 3
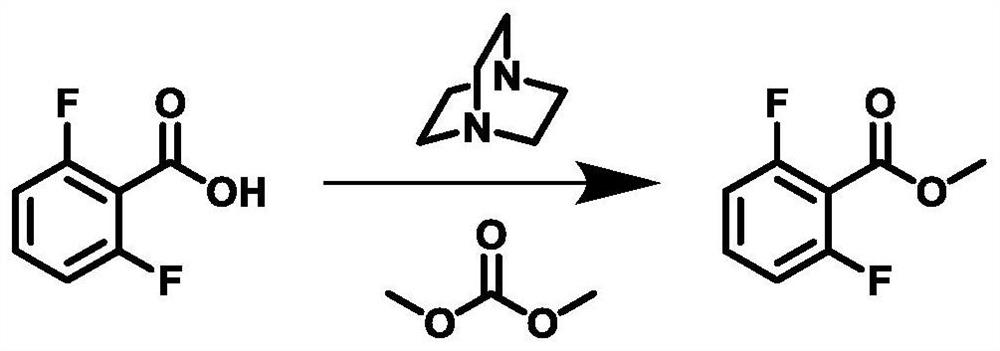
[0044] Method for preparing 2,6-difluorobenzoate methyl ester, specific synthetic route image 3 Indicated.
[0045] 1) 2,6-difluorophenyl (31.6 g, 200 mmol), dimethyl carbonate (305.6 g) and Dabco (22.4 g) were sequentially added to the 1L tetra-neck flask equipped with a stirrer, reflow tube and a thermometer. 200 mmol), stirred at room temperature. The temperature was warmed to 90 ° C, and the insulation reaction was 10 h.
[0046] 2) After the reaction is completed, stop heating, and the reaction is cooled to room temperature. The solvent was evaporated under reduced pressure, and 152.8 g of water was added to the residual liquid, stirred vigorously, extracted with ethyl acetate. Ethyl acetate layer is sequentially with water, dilute hydrochloric acid (5%), saturated sodium hydrogencarbonate, and water, the organic phase is dried and concentrated, that is, 2,6-difluorobenzoate, weight of about 33.3g. 96.8% yield, the content is 98.895%, the result is like Figure 8 Indicated. 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com