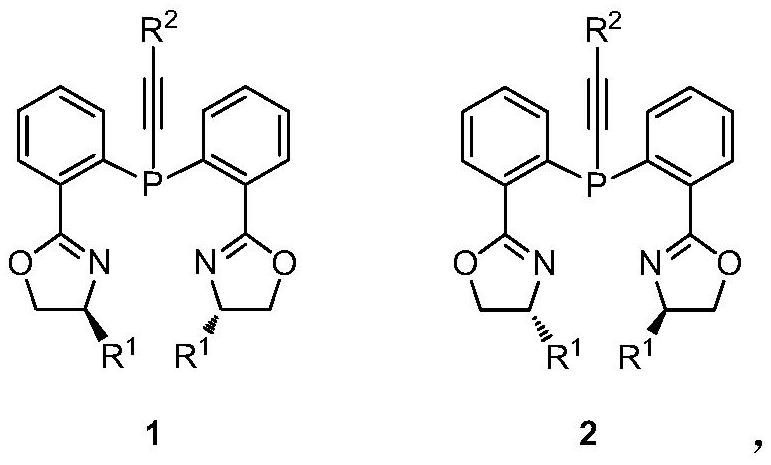
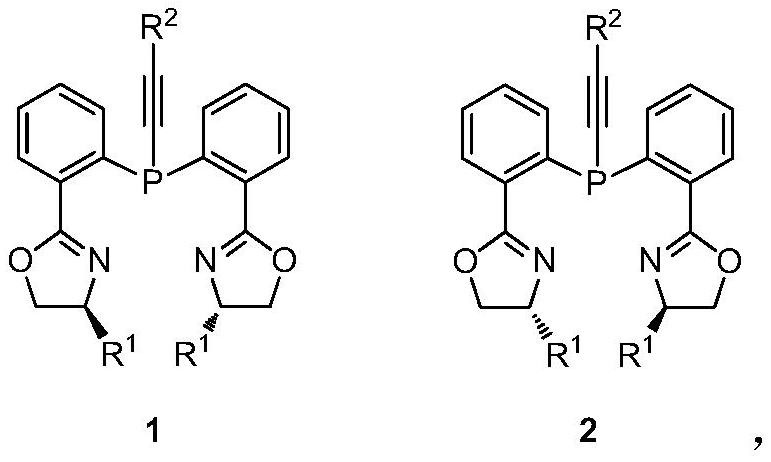
Chiral bisoxazoline-alkynyl phosphine ligand as well as preparation and application thereof
A technology of bisoxazoline and alkynyl phosphine, which is applied to chiral bisoxazoline-alkynyl phosphine ligands and the fields of preparation and application thereof, can solve the problems of ineffective catalytic reaction and high enantioselectivity, and achieves The chiral raw materials are easy to obtain, the chiral raw materials are simple, and the reaction yield is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] Described preparation method comprises the steps:
[0032]
[0033] The 2-(2-bromophenyl)oxazoline 3 used in the present invention was prepared by referring to literature. Specifically, R 1 For isopropyl (Examples 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10) and tert-butyl (Examples 11 and 12), refer to Jin, Y.; Du, D. - M. Tetrahedron, 2012, 68, 3633. When R 1 For phenyl (Example 18), refer to Liu, W.; Ali, S.Z.; Ammann, S.E.; White, M.C.J.Am.Chem.Soc.2018, 140, 10658. When R 1 For cyclohexyl (13, 14 and 15), refer to Tani, K; Behenna, D.C.; McFadden, R.M.; Stoltz, B.M.Org. Lett.2007, 9, 2529. When R1 is methyl (Example 16), refer to Kim, K.S.; Moon, C.W.; Hong, J.K.; Kim, J.H.Bull.Korean.Chem.Soc.2001, 22, 237. When R 1 For ethyl (Example 17), refer to Tang, X.; Zhang, D.; Jie, S.; Sun, W.-H.;
[0034] The terminal alkyne 5 used in the present invention is commercially available.
[0035] Preparation method of the present invention can be further embodied as follows ...
Embodiment 1
[0039] Preparation of chiral bisoxazoline-alkynylphosphine ligand 1a (R1 = isopropyl; R2 = phenyl).
[0040] Yield, 61%; m.p.237.5-239.2°C; 1 H NMR (400MHz, Chloroform-d) δ7.95–7.89(m,3H),7.59–7.53(m,1H),7.51–7.46(m,2H),7.46–7.41(m,2H),7.39–7.34 (m,2H),7.34–7.29(m,3H),4.52–4.26(m,2H),4.16–3.91(m,4H),1.84–1.78(m,1H),1.72–1.59(m,1H) ,1.04(d,J=6.7Hz,3H),0.93(d,J=6.7Hz,3H),0.83(d,J=6.7Hz,3H),0.73(d,J=6.7Hz,3H); 13 C NMR (101MHz, CDCl 3 )δ163.1(d, J=3.0Hz), 162.7(d, J=3.0Hz), 138.9(d, J=19.2Hz), 138.3(d, J=20.2Hz), 134.2, 133.3, 131.72, 131.70 ,131.5(d,J=24.2Hz),131.1(d,J=22.2Hz),130.3,130.3,129.6(d,J=4.0Hz),129.4(d,J=4.0Hz),128.3,128.2,128.1 ,128.0,123.7(d,J=2.0Hz),105.9,90.0(d,J=24.2Hz),73.1,73.0,70.3,70.2,33.0,32.8,19.0,18.8,18.5,18.3. 31 P NMR (162MHz, CDCl 3 )δ-36.6. HRMS (ESI) calcd for C 32 h 33 N 2 o 2 PH([M+H]):509.2352. Found: 509.2355.
Embodiment 2
[0042] Preparation of chiral bisoxazoline-alkynylphosphine ligand 1b (R1 = isopropyl; R2 = methyl).
[0043] Yield, 52%; m.p.222.1-223.8°C; 1 H NMR (400MHz, Chloroform-d) δ7.89–7.66(m,2H),7.48–7.09(m,6H),4.31–4.21(m,1H),4.20–4.13(m,1H),4.05–3.81 (m,4H),1.79–1.61(m,5H),0.98(d,J=6.7Hz,3H),0.92(d,J=6.7Hz,3H),0.87(d,J=6.7Hz,3H) ,0.81(d,J=6.7Hz,3H). 13 C NMR (101MHz, CDCl 3 )δ163.2(d, J=2.0Hz), 162.9(d, J=3.0Hz), 139.4(d, J=19.2Hz), 138.9(d, J=21.2Hz), 133.9, 133.2, 131.4(d ,J=23.2Hz),131.1(d,J=23.2Hz),130.5,130.3,129.5(d,J=4.0Hz),129.4(d,J=3.0Hz),128.1,127.9,103.9,78.2(d ,J=17.2Hz),77.3,73.0,72.9,70.2,70.1,33.0,32.7,19.0,18.8,18.4,18.1,5.7(d,J=2.0Hz). 31 P NMR (162MHz, CDCl 3 )δ-35.8. HRMS (ESI) calcd for C 27 h 31 N 2 o 2 PH([M+H]):447.2196. Found: 447.2189.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com