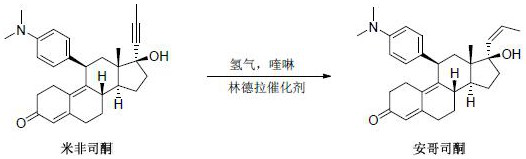
Synthesis method of ancopristone
A synthetic method and technology of angopristone, applied in the field of preparation of steroidal compounds, can solve the problems of difficult industrial production of angopristone synthetic method, achieve the effects of reducing production cost, avoiding preparation, and increasing reaction scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Dissolve mifepristone (50.0 g) in ethanol (500 mL) solution, add Lindella catalyst (15.0 g), add quinoline (10 g), replace hydrogen 3 times, and stir for 6 hours. TLC detects that the reaction is complete, stops the reaction, and filters. The filtrate was spin-dried until it became a paste, and methanol (200 mL) was added to reflux to dissolve, cooled naturally to room temperature, stirred and crystallized, filtered, and dried to obtain 47 grams of light yellow solid powder.
Embodiment 2
[0020] Dissolve mifepristone (1.0 kg) in methanol (8 L) solution, add Lindella catalyst (300.0 g), add quinoline (100 g), replace with hydrogen three times, and stir for 12 hours under 1 atmosphere of hydrogen. TLC detected that the reaction was complete, stopped the reaction, filtered, spin-dried the filtrate to a paste, added methyl tert-butyl ether (5 L) to reflux to dissolve, cooled naturally to room temperature, stirred and crystallized, filtered, and dried to obtain a light yellow solid powder 0.95 kg.
Embodiment 3
[0022] Dissolve mifepristone (30.0 kg) in ether (300 L) solution, add Lindella catalyst (6 kg), add quinoline (2 kg), replace hydrogen 3 times, and stir for 12 hours. TLC detected that the reaction was complete, stopped the reaction, filtered, spin-dried the filtrate to a paste, added methyl tert-butyl ether (120 L) to reflux to dissolve, cooled naturally to room temperature, stirred and crystallized, filtered, and dried to obtain a light yellow solid powder 28.6 kg.
[0023] The H NMR spectrum and carbon spectrum data of gained Angopristone are as follows:
[0024] 1 H NMR (400 MHz, CDCl 3 ) δ 7.02 (d, J = 8.6 Hz, 1H), 6.67 (d, J = 8.2Hz, 1H), 5.74 (s, 1H), 5.65 – 5.55 (m, 1H), 5.50 (d, J = 12.0 Hz, 1H), 4.30(d, J = 7.0 Hz, 1H), 2.91 (s, 6H), 2.78 – 2.66 (m, 1H), 2.56 (m, 2H), 2.51 –2.22 (m, 5H), 2.15 – 1.96 (m, 3H), 1.88 ( d, J = 7.0 Hz, 3H), 1.85 – 1.78 (m,1H), 1.77 – 1.66 (m, 1H), 1.59 – 1.20 (m, 5H), 0.94 – 0.76 (m, 1H), 0.62 (s, 3H);
[0025] 13 C NMR (101 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com