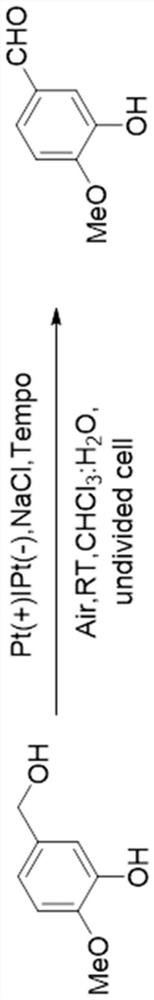
Electrochemical synthesis method of vanillin
A synthesis method and electrochemical technology, which are applied in the electrolysis process, electrolysis components, electrolysis organic production and other directions, can solve the problems of low yield of vanillin, etc., and achieve the effects of simple preparation method, reduced production cost and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take 76.1mg 4-hydroxy-3-methoxybenzyl alcohol, 1.5mL H 2 O, 1.5mL CHCl 3 , 3.9mg TEMPO and 14.6mg NaCl were mixed to obtain solution A, and the obtained solution A was used as the electrolyte, the platinum sheet was used as the cathode, and the platinum sheet was used as the anode for electrolysis to obtain solution B. During the electrolysis process, the current was 5mA, the temperature was 50°C, and the electrolysis time was 15h. Solution B was extracted with 20 mL of ethyl acetate to obtain an organic phase, which was detected by thin layer chromatography (TLC). The obtained organic phase was separated and purified by rotary evaporation (pressure: 400 mmHg, temperature: 40°C, rotary evaporation time: 5 min) and column chromatography (developing solvent: petroleum ether / ethyl acetate = 3:1, volume ratio) Finally, a white solid was obtained, and the yield of vanillin was calculated to be 43%.
Embodiment 2
[0037] Take 1.14g 4-hydroxy-3-methoxybenzyl alcohol, 2mL H 2 O, 2mL CHCl 3 , 60mg TEMPO and 219mgNaCl were mixed to obtain a solution A, and the obtained solution A was used as an electrolyte, the platinum sheet was used as a cathode, and the platinum sheet was used as an anode for electrolysis to obtain a solution B. During the electrolysis process, the current was 5mA, the temperature was 50°C, and the electrolysis time was 24h. Solution B was extracted with 80 mL of ethyl acetate to obtain an organic phase, which was detected by thin layer chromatography (TLC). The obtained organic phase was separated and purified by rotary evaporation (pressure: 400 mmHg, temperature: 40°C, rotary evaporation time: 5 min) and column chromatography (developing solvent: petroleum ether / ethyl acetate = 3:1, volume ratio) Finally, a white solid was obtained, and the yield of vanillin was calculated to be 41%.
Embodiment 3
[0039] Take 11.42g 4-hydroxy-3-methoxybenzyl alcohol, 5mL H 2 O, 5mL CHCl 3 , 780mg TEMPO and 2.19gNaCl were mixed to obtain solution A, and the obtained solution A was used as the electrolyte, the platinum sheet was used as the cathode, and the platinum sheet was used as the anode for electrolysis to obtain solution B. During the electrolysis process, the current was 5mA, the temperature was 50°C, and the electrolysis time was 72h. Solution B was extracted with 500 mL of ethyl acetate to obtain an organic phase, which was detected by thin layer chromatography (TLC). The obtained organic phase was separated and purified by rotary evaporation (pressure: 400 mmHg, temperature: 40°C, rotary evaporation time: 5 min) and column chromatography (developing solvent: petroleum ether / ethyl acetate = 3:1, volume ratio) Finally, a white solid was obtained, and the yield of vanillin was calculated to be 38%.
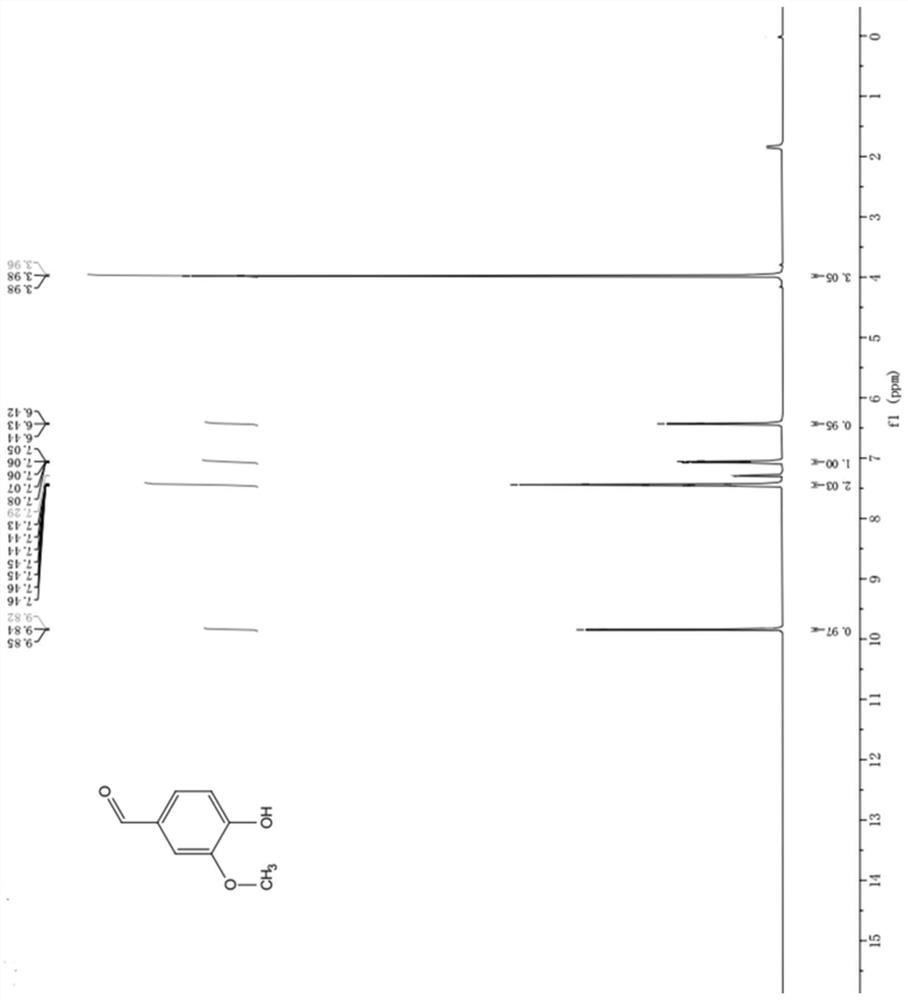
[0040] The product obtained in this embodiment is characterized by NMR, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com