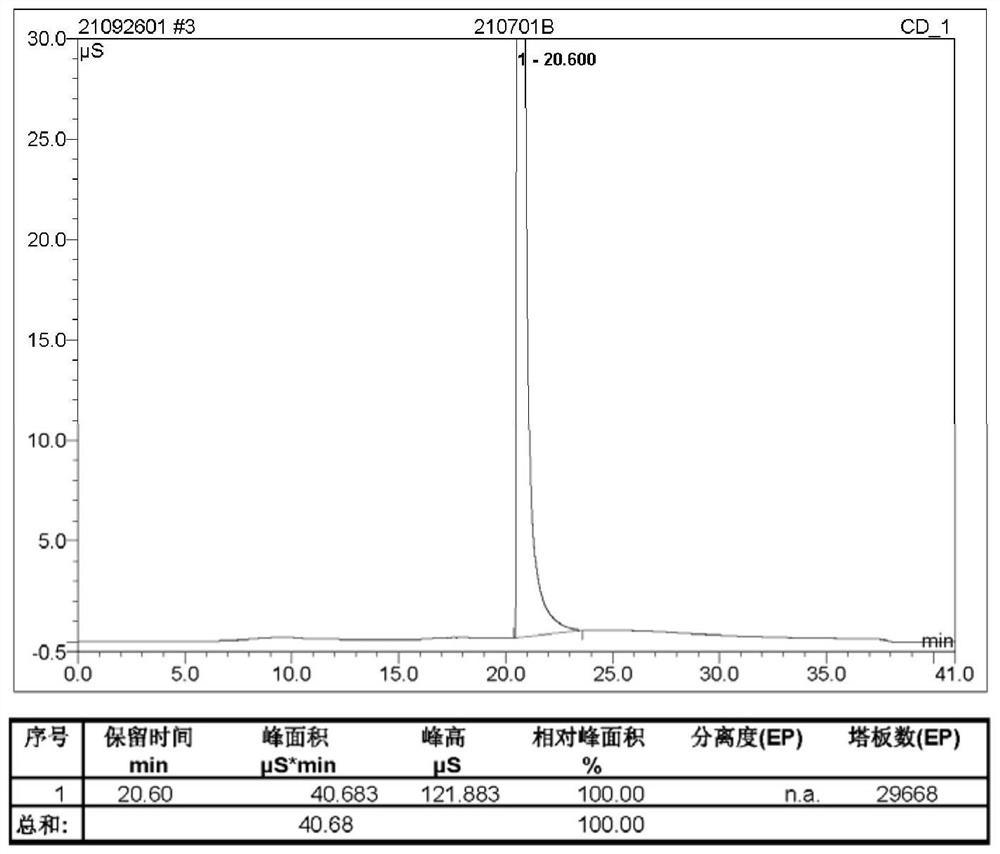
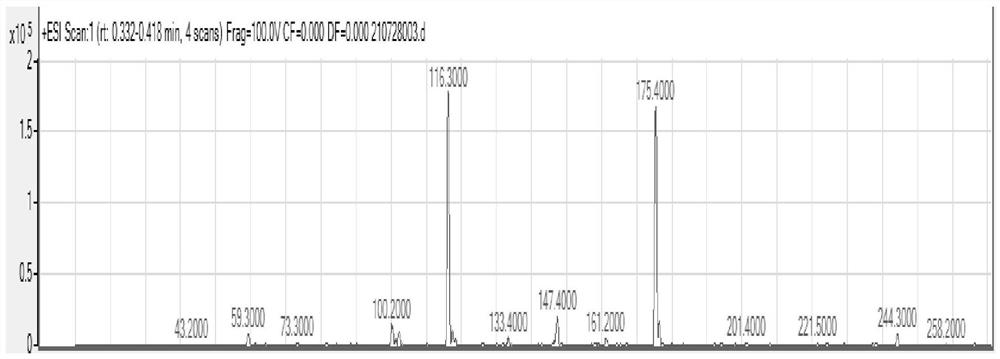
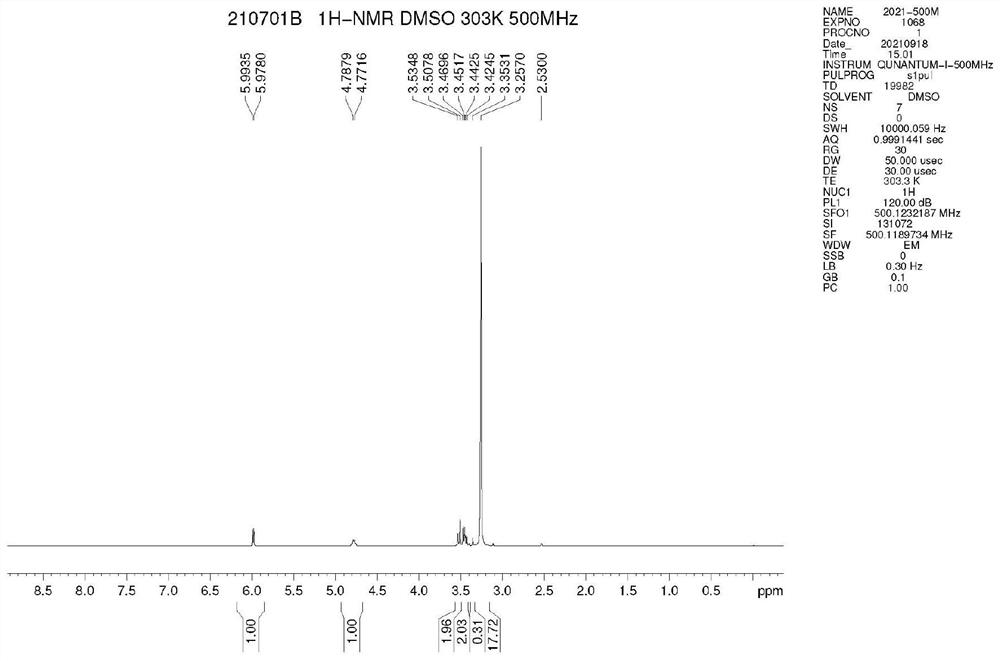
Preparation method of proroiodide
A technology of proammonium iodide and ammonium chloride, which is applied in the field of medicine, can solve the problems of volatile impurities, high toxicity of iodinated reagents, and low yield, and achieve the effects of improving product quality, reducing yield, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The present embodiment provides a kind of preparation route of promonium iodide, and its reaction equation and preparation method are as follows:
[0048]
[0049] (1) Add 310g (1.55mol, 2.0eq) of 30% trimethylamine aqueous solution into a 1L reaction flask, heat up to 40°C, and add dropwise 1,3-dichloro-2-propanol 100g (0.775 mol), the temperature was controlled at 40-45°C, and the reaction was continued at this temperature for 5 hours after dropping.
[0050] After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain an off-white solid. Add 200g of methanol, reflux and dissolve at 82±2°C, cool to 2±2°C for crystallization. After suction filtration, the filter cake was vacuum-dried at 45° C. to obtain 160 g of a white solid (crude ammonium chloride intermediate) with a yield of 84%.
[0051] Recrystallize 160 g of the intermediate ammonium chloride crude product with 240 g of methanol, decolorize with activated carbon, filter...
Embodiment 2
[0055] The present embodiment provides a kind of preparation route of promonium iodide, and its reaction equation and preparation method are as follows:
[0056]
[0057] (1) Add 460g (2.33mol, 3.0eq) of 30% trimethylamine aqueous solution into a 1L reaction flask by weight, raise the temperature to 50°C, and add 100g (0.775mol) of 1,3-dichloro-2-propanol dropwise under stirring. ), the temperature was controlled at 50-55° C., and the dropwise reaction was continued at this temperature for 2 hours.
[0058] After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain an off-white solid. Add 300g of ethanol, reflux and dissolve at 82±2°C, cool to 2±2°C for crystallization. After suction filtration, the filter cake was vacuum-dried at 45° C. to obtain 156 g of a white solid (crude ammonium chloride intermediate), with a yield of 82%.
[0059] 156 g of the intermediate ammonium chloride crude product was recrystallized with 320 g of ethan...
Embodiment 3
[0063] The present embodiment provides a kind of preparation route of promonium iodide, and its reaction equation and preparation method are as follows:
[0064]
[0065] (1) Add 920g (4.66mol, 5eq) of 30% trimethylamine aqueous solution to the 2L reaction flask by weight, raise the temperature to 65°C, add 100g (0.775mol) of 1,3-dichloro-2-propanol dropwise under stirring , the temperature was controlled at 65-70°C, and the mixture was stirred for another 30 minutes after dropping.
[0066] After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain an off-white solid. Add 300g of isopropanol, reflux and dissolve at 82±2°C, cool to 2±2°C for crystallization. After suction filtration, the filter cake was vacuum-dried at 45° C. to obtain 174 g of a white solid (crude ammonium chloride intermediate), with a yield of 91%.
[0067] Recrystallize 174 g of the intermediate ammonium chloride crude product with 350 g of isopropanol, decoloriz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com