Synthesis method and application of chiral alpha-propargyl-3-indole compound and derivative thereof
A technology of propargyl and compounds, which is applied in the field of synthesis of chiral α-propargyl-3-indole compounds and derivatives thereof, can solve the problem of poor selectivity, lack of structural diversity and limited application range of substrates and other problems, to achieve the effect of high selectivity, wide substrate applicability, and good inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
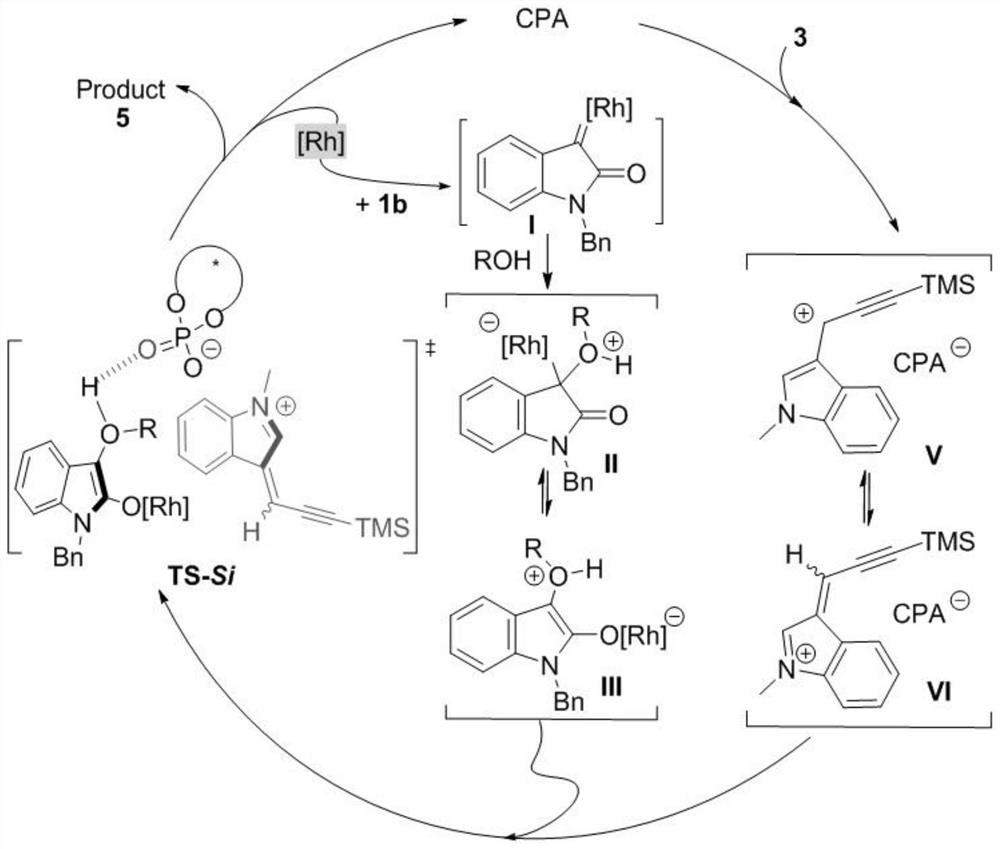
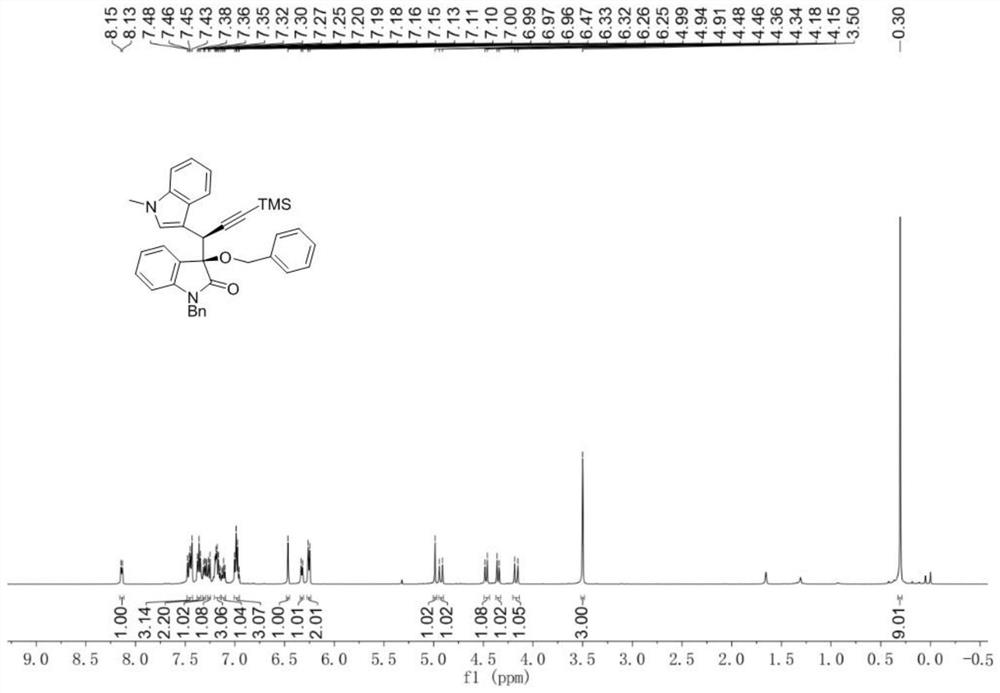
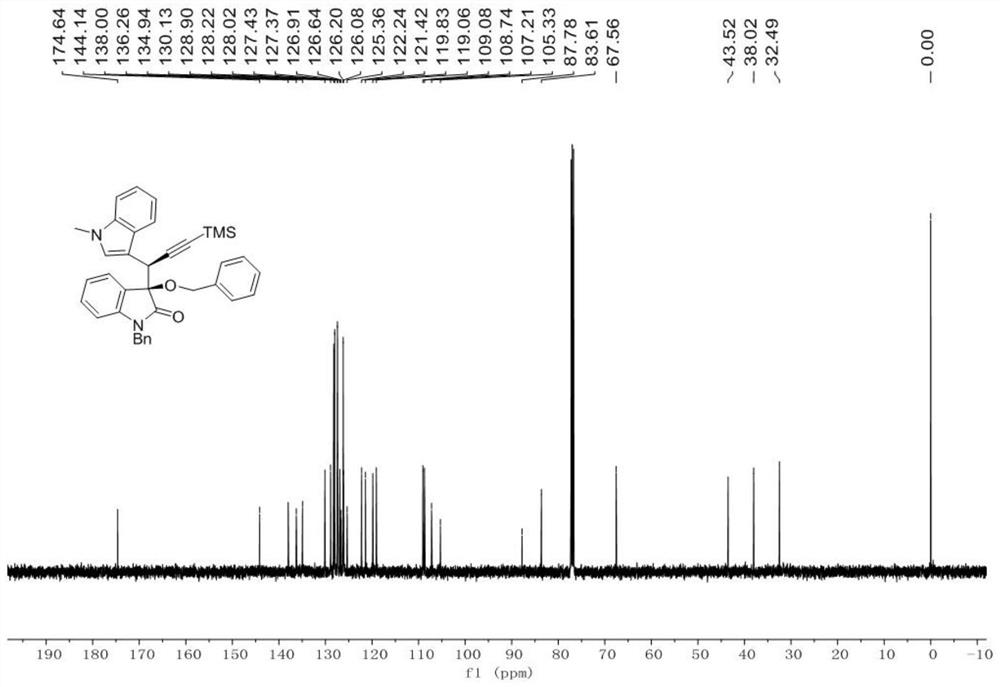
[0072] Embodiment 1 A kind of chemical synthesis method of chiral α-propargyl-3-indole compounds and derivatives thereof
[0073] According to reaction formula (II), with the diazo compound of formula (1), the alcohol of formula (2) and the indole propargyl alcohol of formula (3) as raw material, Rh 2 (esp) 2 and chiral phosphoric acid as a catalyst, Molecular sieves are water-absorbing additives. After a one-step three-component reaction in an organic solvent, the product chiral α-propargyl-3-indole compounds or their derivatives (syn-5 and anti-5) can be obtained with high selectivity:
[0074]
[0075] In the reaction formula, Ar 1 independently selected from phenyl, 5-methylphenyl, 5-chlorophenyl, 6-chlorophenyl, 6-bromophenyl, 6-methoxyphenyl, 7-methylphenyl, naphthyl, 2-methylnaphthyl or 2-methoxynaphthyl; Ar 2 independently selected from phenyl, 5-chlorophenyl, 5-methoxyphenyl, 6-methoxyphenyl, 7-methylphenyl, naphthyl, 2-methylnaphthyl, 2-methoxy Base naphthyl...
Embodiment 2
[0157] Example 2 Antitumor activity test of chiral α-propargyl-3-indole compounds and derivatives thereof
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com