Antigen peptide of DNA methyltransferase 1 and polyclonal antibody thereof
A polyclonal antibody, methyltransferase technology, applied in the direction of transferase, genetic engineering, plant genetic improvement, etc., can solve the problems of low specificity and complex analysis process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Amplification of pig DNMT1 gene and construction of eukaryotic expression vector
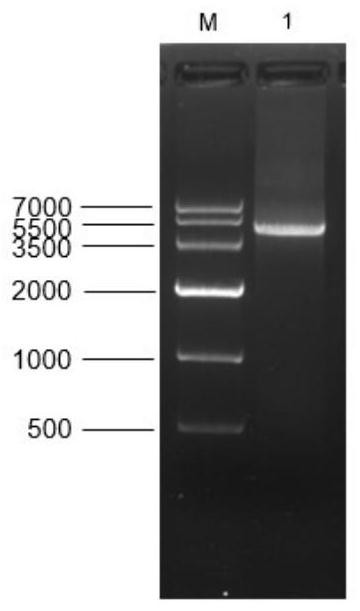
[0031] Primers were designed according to the predicted sequence of the porcine DNMT1 gene (XM_021082029.1) in GenBank, the RNA was reverse-transcribed into cDNA, and the cDNA was used as a template for PCR amplification. The size of the fragment band was consistent with the expected size.
[0032] The pCMV-Flag14 plasmid and DNMT1 gene were double digested and ligated using BamHI and HindIII respectively, and then verified by sequencing that the pFlag14-DNMT1 (cloned) recombinant plasmid had been successfully constructed, wherein the amino acid sequence of the predicted sequence of the porcine DNMT1 gene As shown in SEQ ID NO.3, its nucleotide sequence is shown in SEQ ID NO.4. figure 1 It is the porcine DNMT1 gene identification result (5102bp) that utilizes RT-PCR method to amplify from alveolar macrophage in the example of the present invention; Wherein, M: DNA Maker; Swim...
Embodiment 2
[0033] Example 2 Preparation of porcine DNMT1 polyclonal antibody
[0034] As shown in SEQ ID NO.1, the polypeptide fragment with the amino acid sequence of SSPVKRPRKEPVDED was selected as the antigenic peptide of the polyclonal antibody, coupled to the KLH carrier protein for purification, and then the antigenic peptide was diluted with physiological saline, and the ratio was 1:1 Mix and emulsify with Freund's adjuvant to immunize New Zealand white rabbits. Before the formal immunization, venous blood collection is required as a negative control. The immunization method is subcutaneous injection or intramuscular injection. The total amount of antigen is 500ug. Complete adjuvant booster immunization once, 7 days after immunization six times to obtain polyantiserum by venous or cardiac blood collection. The titer of the polyclonal antibody determined by the indirect ELISA method is greater than 1:50k, which can ensure that the polyclonal antibody can be used for Western Blot de...
Embodiment 3
[0035] The specificity analysis of embodiment 3 polyclonal antibody
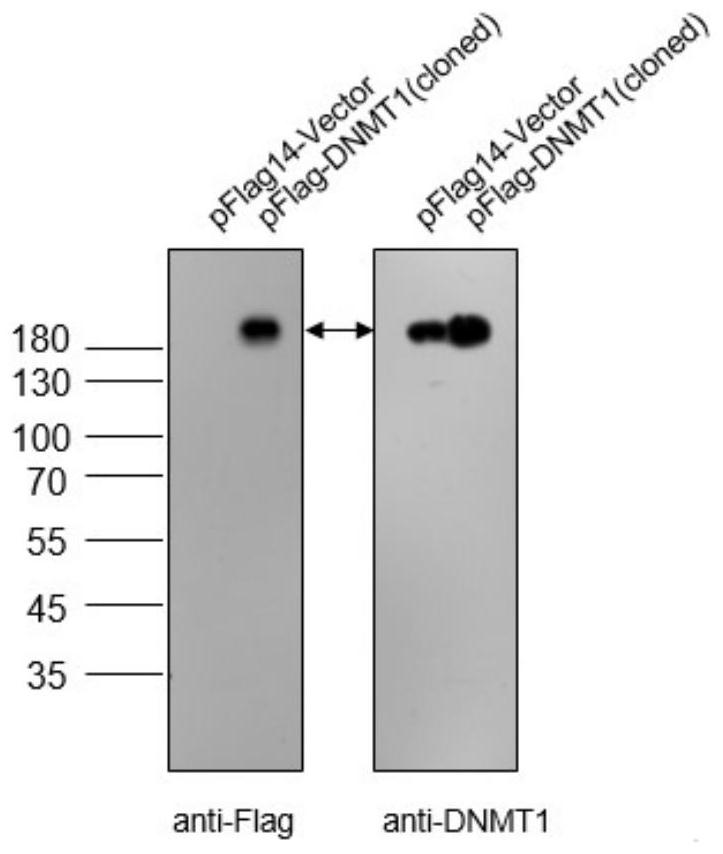
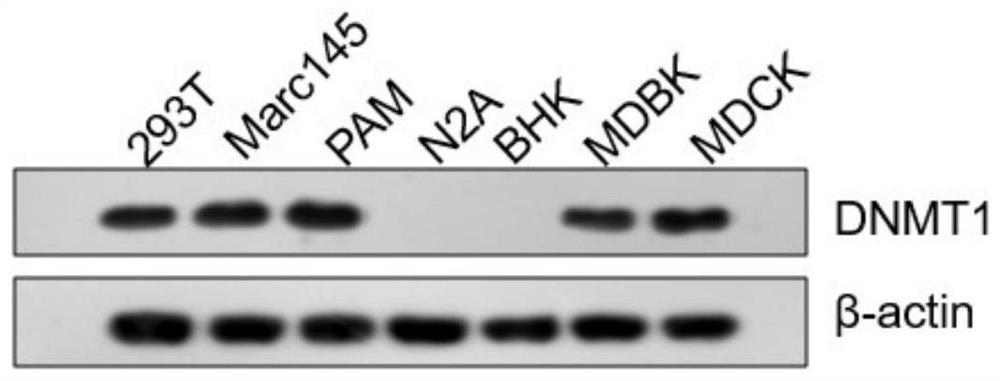
[0036]In order to verify whether the obtained DNMT1 protein synthetic peptide polyclonal antibody can be used in subsequent Western Blot experiments, the obtained DNMT1 protein synthetic peptide polyclonal antibody was used to detect the overexpression of pFlag-DNMT1 (cloned) plasmid in HEK 293T cells, by using Mouse anti-Flag antibody and rabbit anti-DNMT1 polyclonal antibody were used as primary antibodies for detection, figure 2 It is the protein result chart expressed by the recombinant plasmid pFlag14-DNMT1 (cloned), such as image 3 As shown, both antibodies were able to display the overexpressed DNMT1 protein band, and the expected size was consistent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com