Synthesis method of 3-aminopyrrolidine dihydrochloride
A technology of aminopyrrolidine dihydrochloride and aminopyrrolidine, which is applied in the field of synthesis of 3-aminopyrrolidine dihydrochloride, and can solve problems such as many steps, long reaction routes, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
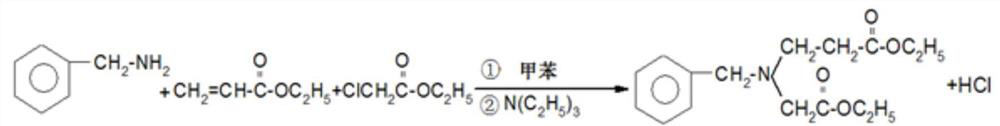
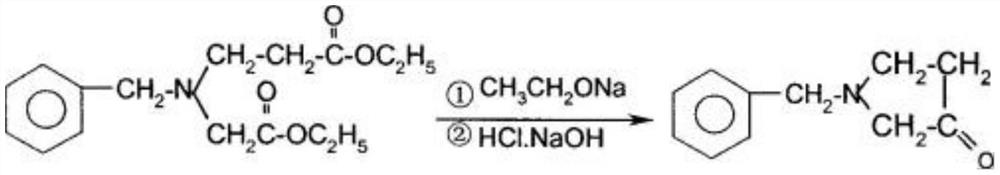
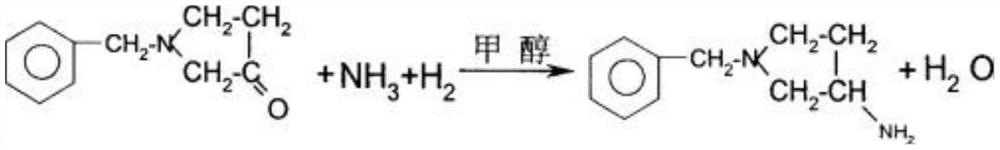
[0073] This embodiment provides a method for synthesizing 3-aminopyrrolidine dihydrochloride, comprising the following steps:
[0074] 1) Preparation of benzyl-(3-ethoxy-3-enyl)-(1-vinylethoxymethyl)amine liquid;
[0075] (1) Use a vacuum pump to sequentially draw toluene (recoverable for mechanical use) and benzylamine into the reaction tank; the interlayer passes through cooling water or cold brine;
[0076] (2) Under stirring, the ethyl acrylate is sucked into the reaction tank in stages, and after the feeding is completed, the temperature is naturally raised to be stable, and the stirring is continued for 10-15 hours;
[0077] (3) Within 40°C, fill in 79.0kg of hydrogen chloride, after the feeding is completed, continue to stir and react for 4-6 hours;
[0078] (4) Open centrifuge, centrifugal separation; Filter cake is washed with toluene; All filtrates are merged into retort; Inhale ethyl chloroacetate, triethylamine;
[0079] (5) After the pumping is completed, the te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com