6-hydroxy-3-hexenylalkoxymethyl ether compound and method for preparing 3, 13-octadecadiene-1-ol from 6-hydroxy-3-hexenylalkoxymethyl ether compound
A technology of hexenyl alkoxymethyl ether and octadecadiene, applied to 6-hydroxy-3-hexenyl alkoxymethyl ether compound and preparing 3,13-octadecadiene- In the field of 1-alcohol, it can solve problems such as difficult prevention and control, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
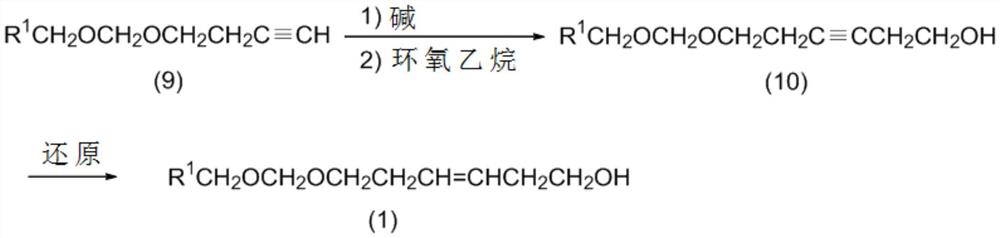
[0301] Embodiment 1: prepare 6-hydroxyl-3-hexynyl methoxymethyl ether (10:R 1 =H)
[0302]
[0303] Methylmagnesium chloride (366.84 g, 4.91 mol) and tetrahydrofuran (1530.16 g) were placed in a reactor at room temperature and stirred at 20°C to 25°C for 4 minutes. After the stirring is completed, 3-butynylmethoxymethyl ether (9:R 1 =H) (517.25 g, 4.50 mol, 99.30% purity) was added dropwise to the reactor. After the dropwise addition was complete, the reaction mixture was stirred at 60°C to 70°C for 5 hours. Subsequently, ethylene oxide (257.69 g, 5.85 mol) was added dropwise at 40°C to 60°C. After the dropwise addition was complete, the reaction mixture was stirred at 60°C to 65°C for 3.5 hours. Next, a solution of acetic acid (800.00 g) in water (1600.00 g) was added to the reaction mixture, followed by phase separation. The aqueous phase was removed to obtain an organic phase. The organic phase thus obtained was concentrated under reduced pressure, and the concentr...
Embodiment 2
[0308] Embodiment 2: preparation (3Z)-6-hydroxyl-3-hexenyl methoxymethyl ether (1:R 1 =H)
[0309]
[0310] At room temperature, the 6-hydroxyl-3-hexynyl methoxymethyl ether (10:R) obtained in Example 1 was 1 =H) (635.95 g, 3.91 mol, purity 97.16%) and P-2Ni catalyst (108.60 g) were placed in the reactor. The reactor was purged with hydrogen at 45°C to 55°C for 7.5 hours with stirring. After confirming that the conversion rate was 100%, water (170.94 g) was added to the reaction mixture, followed by phase separation. The aqueous phase was removed to obtain an organic phase. The organic phase thus obtained was concentrated under reduced pressure, and the concentrate was distilled under reduced pressure to obtain (3Z)-6-hydroxy-3-hexenylmethoxymethyl ether (1:R 1 =H) (612.16g, 3.62mol, purity 94.74%, b.p.=107.2°C to 111.0°C / 0.40kPa (3.0mmHg)), yield 92.58%.
[0311] The following is thus prepared (3Z)-6-hydroxy-3-hexenyl methoxymethyl ether (1:R 1 =H) spectral data.
...
Embodiment 3
[0315] Embodiment 3: preparation (3E)-6-hydroxyl-3-hexenyl methoxymethyl ether (1:R 1 =H)
[0316]
[0317]Lithium aluminum hydride (42.50 g, 1.12 mol) and diethylene glycol dimethyl ether (666.24 g) were placed in a reactor at room temperature, and stirred at 50°C to 55°C for 15 minutes. After the stirring was completed, the 6-hydroxy-3-hexynyl methoxymethyl ether (260.85 g, 1.60 mol, purity 97.03%) obtained according to Example 1 was added dropwise at 50°C to 60°C, and at 130°C to 135°C °C and stirred for 20 hours. After cooling to 20°C to 25°C, tetrahydrofuran (2508.78g), water (42.50g), sodium hydroxide (0.16mol) aqueous solution (170.03g) and diatomaceous earth (Celite) (529.51g) were added successively, and stirred for 12 Hour. After the stirring was completed, the reaction mixture was filtered to obtain an organic phase. The organic phase thus obtained was concentrated under reduced pressure, and the concentrate was distilled under reduced pressure to obtain (3E)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com