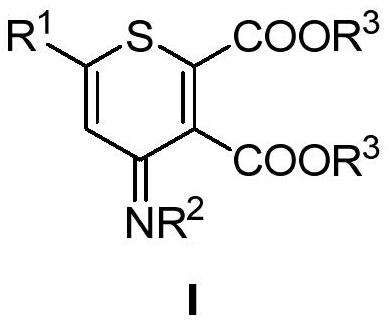
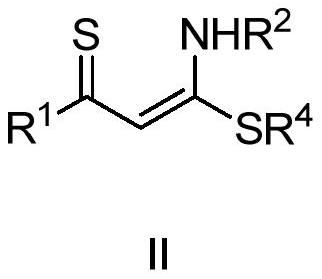
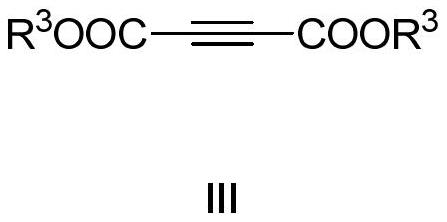
Polysubstituted thiapyran derivative and synthesis method thereof
A synthetic method and a multi-substituted technology are applied in the field of multi-substituted thiopyran derivatives and their synthesis to achieve the effects of wide applicability, good stereoselectivity and high yield of target products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] In the glove box, weigh 1-methylthio-1-benzylamine-1-butene-3-phenyl-3-thione 2a (0.3mmol), butynedioic acid dimethyl ester 3 (0.45mmol ), zinc chloride (0.03mmol) in a 25mL Schlenk reaction flask, under nitrogen, add DMF 2mL, put into 120 ℃ oil bath and react for 12 hours. After the reaction, the mixture was cooled to room temperature, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=20:1 ), to obtain the yellow liquid target product 1a (92 mg, yield 81%). The target product was confirmed by NMR and high-resolution mass spectrometry.
[0042] Compound Characterization Data
[0043] 6-Phenyl-4-anilino-4hydro-thiopyran derivative (1a), yellow solid. 1 H NMR (400MHz, CDCl 3 )δ7.01(m,2H),6.92(m,4H),6.63-6.45(m,5H),3.69(s,3H),3.63(s,3H). 13 C{ 1 H}NMR (100MHz, CDCl 3 )δ162.0, 157.0, 151.3, 150.4, 140.1, 138.3, 135.2, 131.0, 126.9,...
Embodiment 2
[0045]
[0046] In the glove box, weigh 1-methylthio-1-p-toluidine-1-butene-3-o-bromophenyl-3-thione 2b (0.3mmol), dimethyl butyndioate 3 (0.45mmol), zinc chloride (0.03mmol) in a 25mL Schlenk reaction flask, under nitrogen, add DMF 2mL, and put it in an oil bath at 120°C for 12 hours. After the reaction, the mixture was cooled to room temperature, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=20:1 ), to obtain the yellow liquid target product 1b (108mg, yield 85%). The target product was confirmed by NMR and high-resolution mass spectrometry.
[0047] Compound Characterization Data
[0048] 6-o-methylphenyl-4-p-methoxyanilino-4hydro-thiopyran derivative (1b), yellow solid. 1 H NMR (400MHz, CDCl 3 )δ7.01(m,1H),6.90(m,3H),6.61-6.44(m,5H),3.69(s,3H),3.62(s,3H),3.46(s,3H),2.00(s ,3H). 13 C{ 1 H}NMR (100MHz, CDCl 3 C 23 h 21 NO 5 HRMS theore...
Embodiment 3
[0050]
[0051] In the glove box, successively weigh 1-ethylthio-1-ethylamine-1-butene-3-naphthyl-3-thione 2c (0.3mmol), butyndioic acid di-tert-butyl ester 4 ( 0.45mmol) (Aldrich CAS: 66086-33-7), zinc chloride (0.03mmol) in a 25mL Schlenk reaction flask, under nitrogen, add DMF 2mL, and put it in an oil bath at 120°C for 12 hours. After the reaction, the mixture was cooled to room temperature, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=20:1 ), the target product 1c (74 mg, yield 53%) was obtained as a yellow liquid. The target product was confirmed by NMR and high-resolution mass spectrometry.
[0052] Compound Characterization Data
[0053] 6-Naphthyl-4-ethylamino-4hydro-thiopyran derivative (1c), yellow liquid. 1 H NMR (400MHz, CDCl 3 )δ7.05(m,1H),6.94(m,2H),6.65-6.40(m,4H),4.11(q,J=7.1Hz,2H),1.55(s,9H),1.45(s,9H ), 1.09(t,J=7.1Hz,3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com