Terazosin hydrochloride capsule and preparation method thereof
A technology of terazosin hydrochloride and terazosin, applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as poor fluidity, poor uniformity of powder mixing, and low dissolution rate of finished products within 15 minutes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation of the contents of each batch of capsules, wherein the terazosin hydrochloride raw material particle size D90 is different, the specific operation: sieve the obtained raw material drug to obtain the raw material drug with different particle sizes; the excipients are all from the same batch of materials , Lactose is lactose monohydrate, D90 is 119.53um; microcrystalline cellulose is 102 type, D90 is 149.98um; crospovidone D90 is 119.81um; magnesium stearate does not need to control its particle size due to its small amount .
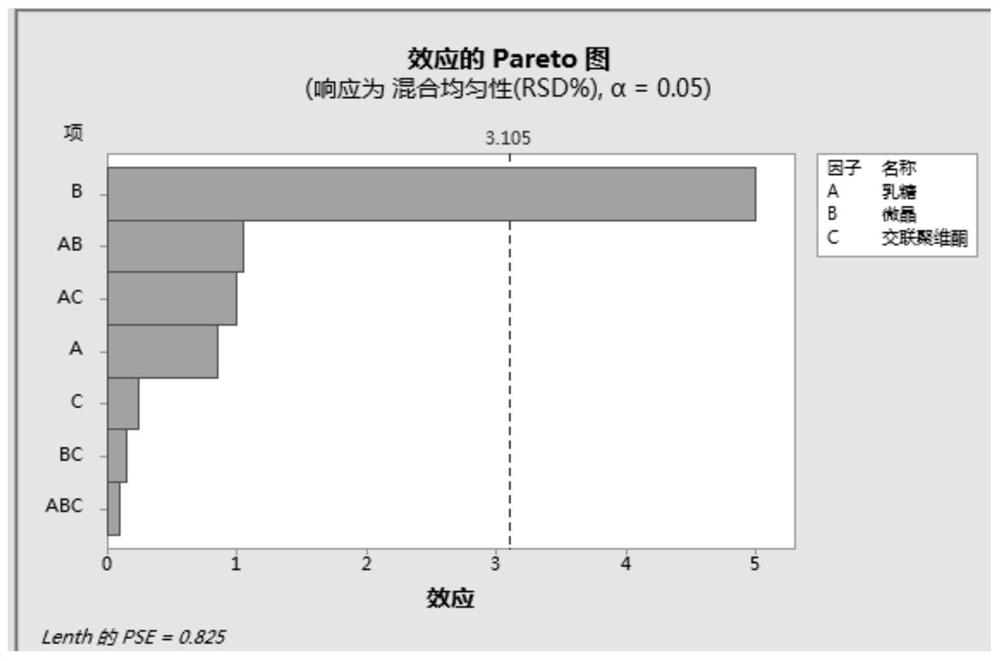
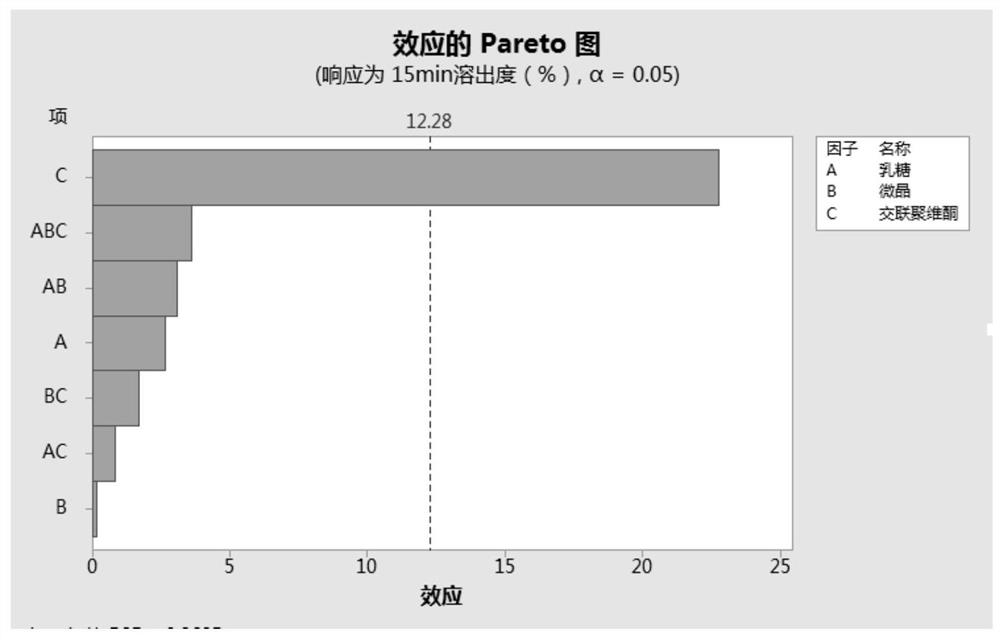
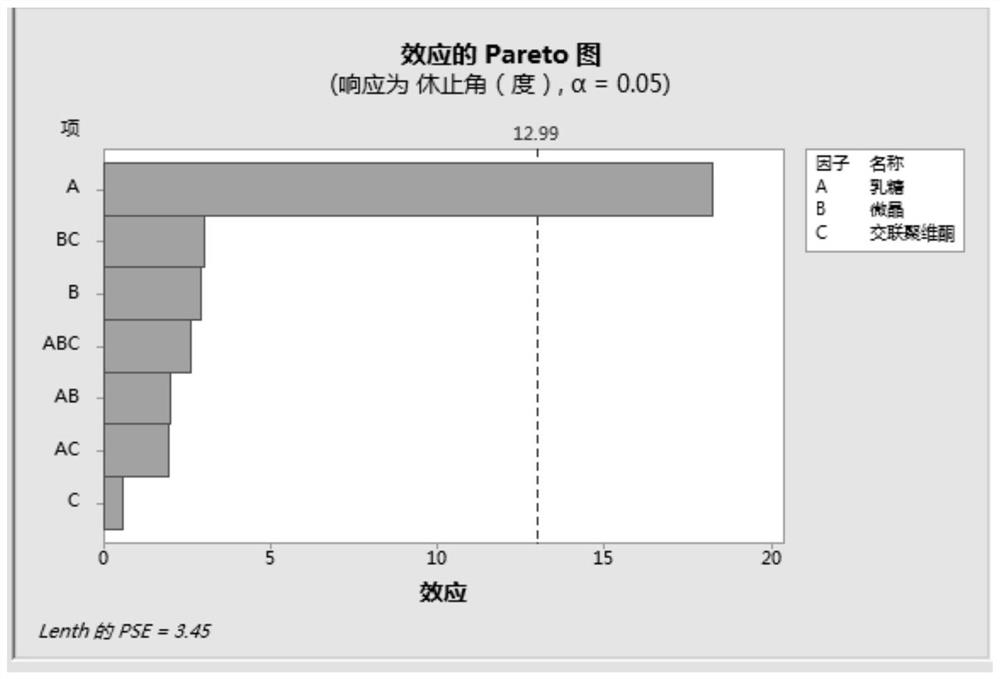
[0047] Since the bulk drug of this product is highly soluble, and there is no significant difference in the dissolution rate of different particle sizes under the four media, the dissolution test of the finished product is not carried out in the investigation of the particle size of the bulk drug, and the focus is on the dissolution of the terazosin hydrochloride bulk drug of different particle sizes in the mixture. Mixing uniformit...
Embodiment 1
[0064] Terazosin hydrochloride capsules, made of the following weight ratio: 2 parts of terazosin hydrochloride (in terms of terazosin), 178 parts of lactose monohydrate, 28 parts of microcrystalline cellulose, 14 parts of crospovidone 3 parts, magnesium stearate 3 parts.
[0065] Preferably, the particle size of lactose monohydrate is controlled so that D90 is 119.53um;
[0066] The microcrystalline cellulose is a 102 model, and the D90 is 149.98um; the crospovidone is an XL model, and the D90 is 100.22um.
[0067] Preparation Process:
[0068] (1) Pass the terazosin hydrochloride bulk drug through a 60-mesh sieve, and set aside.
[0069] (2) Weigh spare terazosin hydrochloride crude drug, lactose monohydrate, crospovidone, and microcrystalline cellulose.
[0070] (3) Add the materials weighed in step (2) into the mixer in the following order, and then add about half of the prescription amount of lactose, crospovidone, terazosin raw material medicine, microcrystalline cell...
Embodiment 2
[0075] Terazosin hydrochloride capsules, made of the following weight ratio: 2 parts of terazosin hydrochloride, 160 parts of lactose monohydrate, 31 parts of microcrystalline cellulose, 16 parts of crospovidone, 2.7 parts of magnesium stearate .
[0076] Preferably, the particle size of lactose monohydrate is controlled so that D90 is 119.53um;
[0077] The microcrystalline cellulose is a 102 model, and the D90 is 149.98um; the crospovidone is an XL model, and the D90 is 100.22um.
[0078] Preparation Process:
[0079] (1) Pass the terazosin hydrochloride bulk drug through a 60-mesh sieve, and set aside.
[0080] (2) Weigh spare terazosin hydrochloride crude drug, lactose monohydrate, crospovidone, and microcrystalline cellulose.
[0081] (3) Add the materials weighed in step (2) into the mixer in the following order, and then add lactose, crospovidone, terazosin hydrochloride bulk drug, microcrystalline cellulose and The remaining prescription amount of lactose was mixed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com