Method for preferentially adsorbing and separating ethylbenzene from C8 aromatic hydrocarbon isomeride mixture
A technology of isomers and C8 aromatics, which is applied in the field of preferential adsorption and separation of ethylbenzene, can solve the problems of poor separation ability and low adsorption selectivity, and achieve weak adsorption force, cheap raw materials, and easy desorption cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Weigh 5g Co(NO 3 ) 2 Dissolve in 50ml of N,N-dimethylformamide, then measure 5ml of formic acid into the solution. Stir and heat the above mixture to 100°C for 12 to 24 hours to obtain the product [Co 3 (HCOO) 6 ] was filtered and washed with methanol, and then activated in vacuum at a temperature of 25 to 100°C for 2 to 12 hours to obtain the activated [Co 3 (HCOO) 6 ]Material.
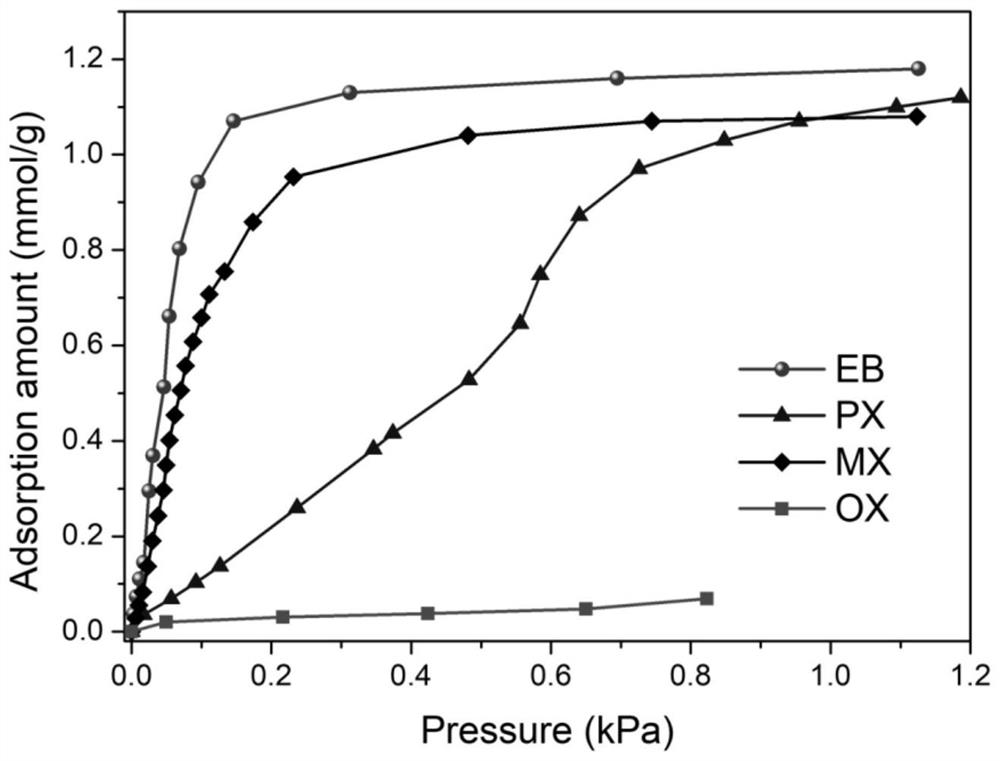
[0044] [Co 3 (HCOO) 6 ] The adsorption isotherms of p-xylene isomers and ethylbenzene at 298K are as follows figure 1 shown.
Embodiment 2
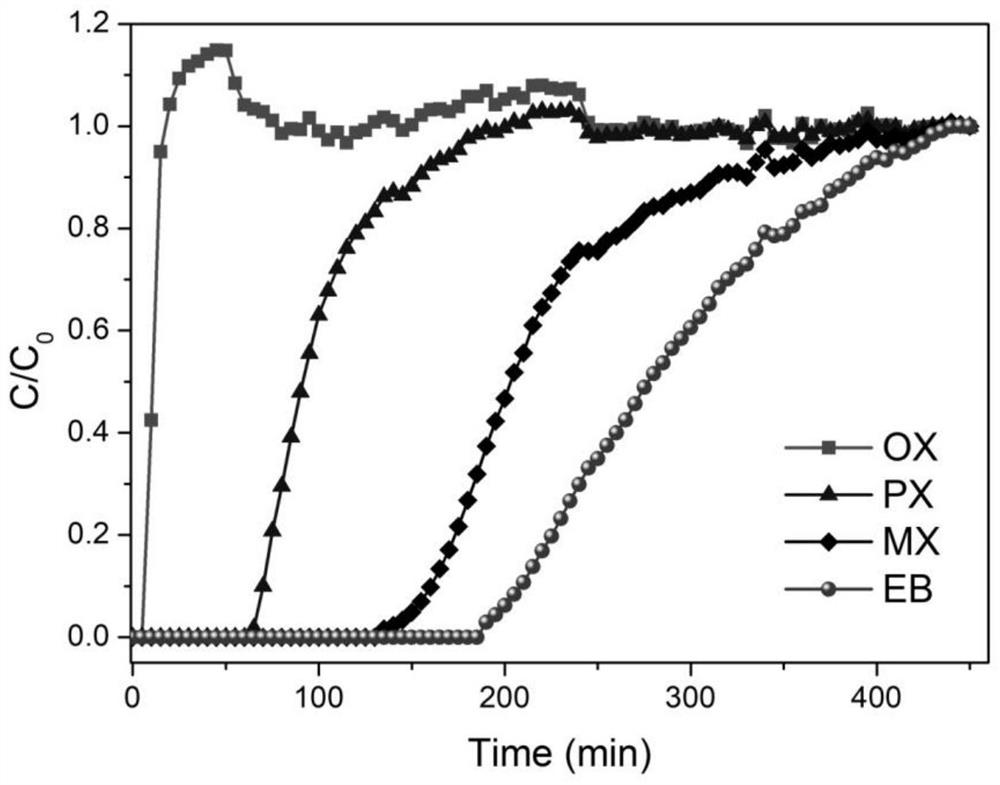
[0046] [Co prepared by embodiment 1 3 (HCOO) 6 ] pack into 5cm adsorption column, adopt nitrogen bubbling mode to obtain the mixed gas containing p-xylene, m-xylene, o-xylene, ethylbenzene (mass ratio is 1:1:1:1), then mixed gas with 10 ~20mL / min flow rate into the adsorption column, the operating temperature of the adsorption column is 25 degrees Celsius, the breakthrough data curve is as follows figure 2 As shown, high-purity o-xylene gas can be obtained in the effluent gas, and high-purity ethylbenzene gas can be obtained after the adsorption column is desorbed at 50-150 degrees Celsius.
Embodiment 3
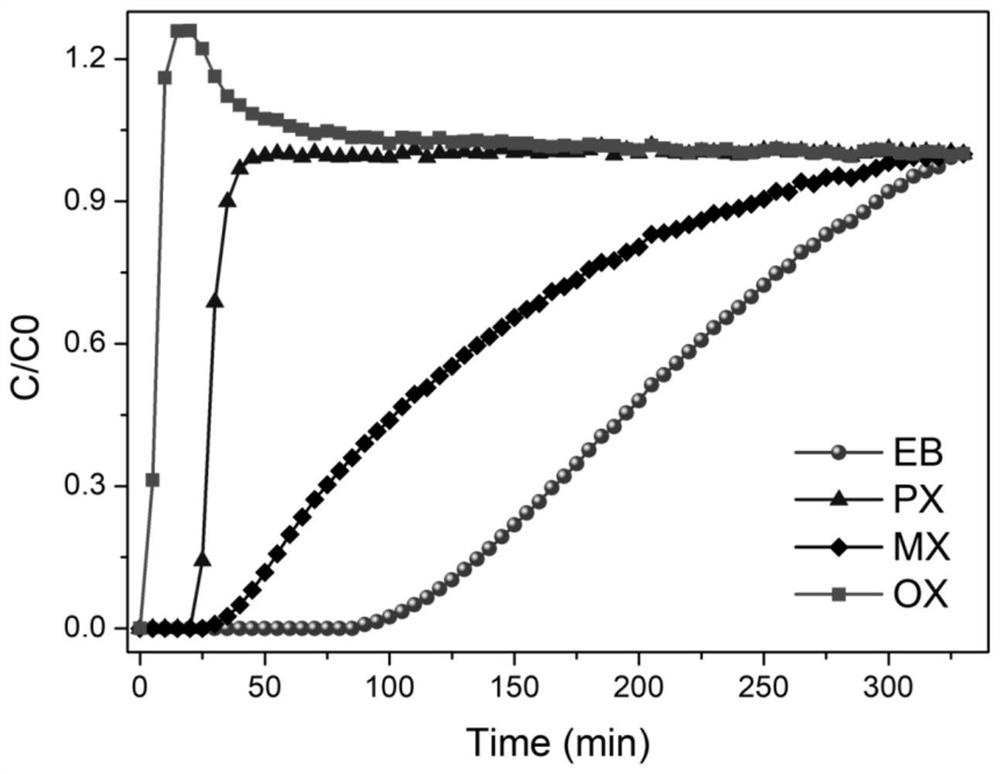
[0048] Nitrogen bubbling is used to obtain a four-component mixed gas containing p-xylene, m-xylene, o-xylene, and ethylbenzene (mass ratio 1:1:1:1), and then the mixed gas is mixed at 20-40mL / min The flow rate is passed into the adsorption column in Example 2, the operating temperature of the adsorption column is 60 degrees Celsius, and the breakthrough data curve is as follows image 3 As shown, high-purity o-xylene gas can be obtained in the effluent gas, and high-purity ethylbenzene gas can be obtained after the adsorption column is desorbed at 50-150 degrees Celsius.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com