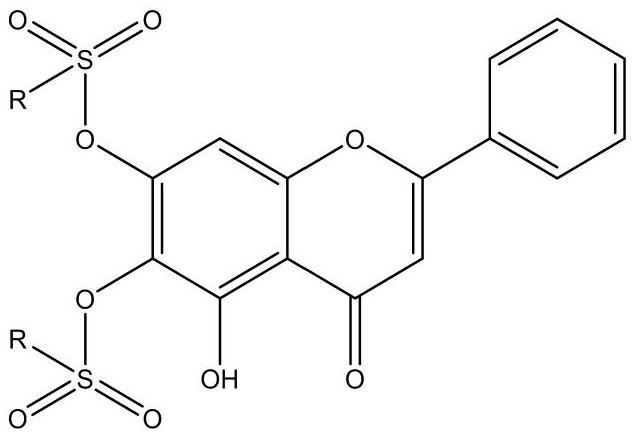
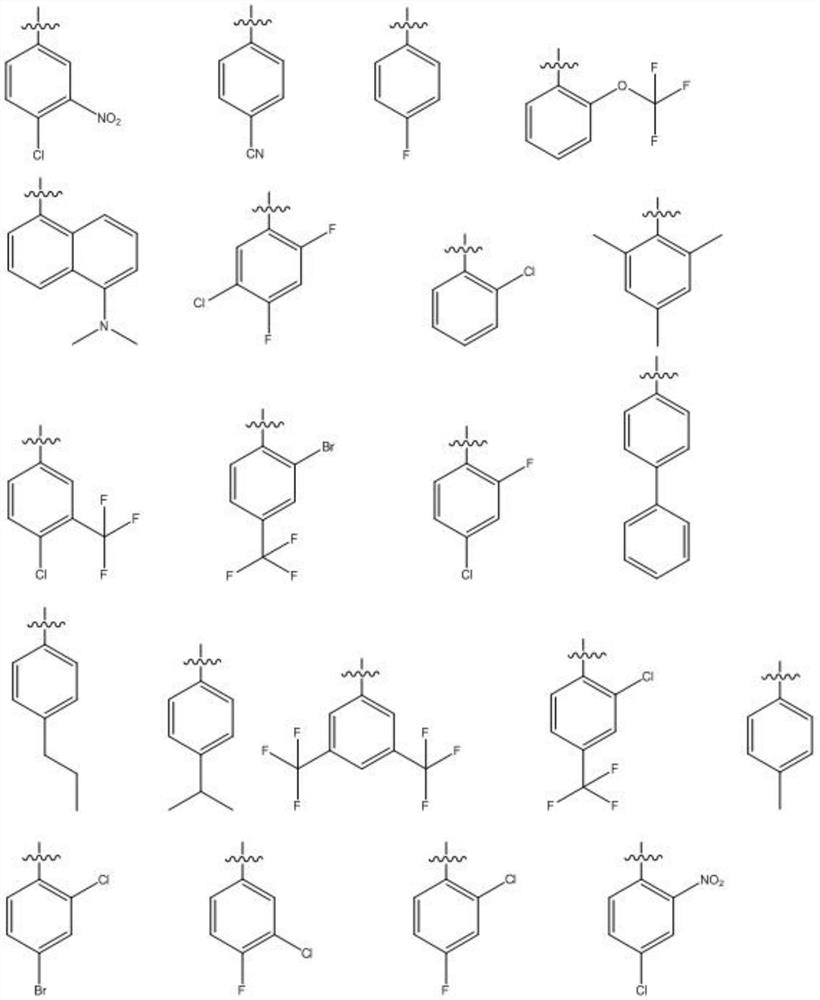
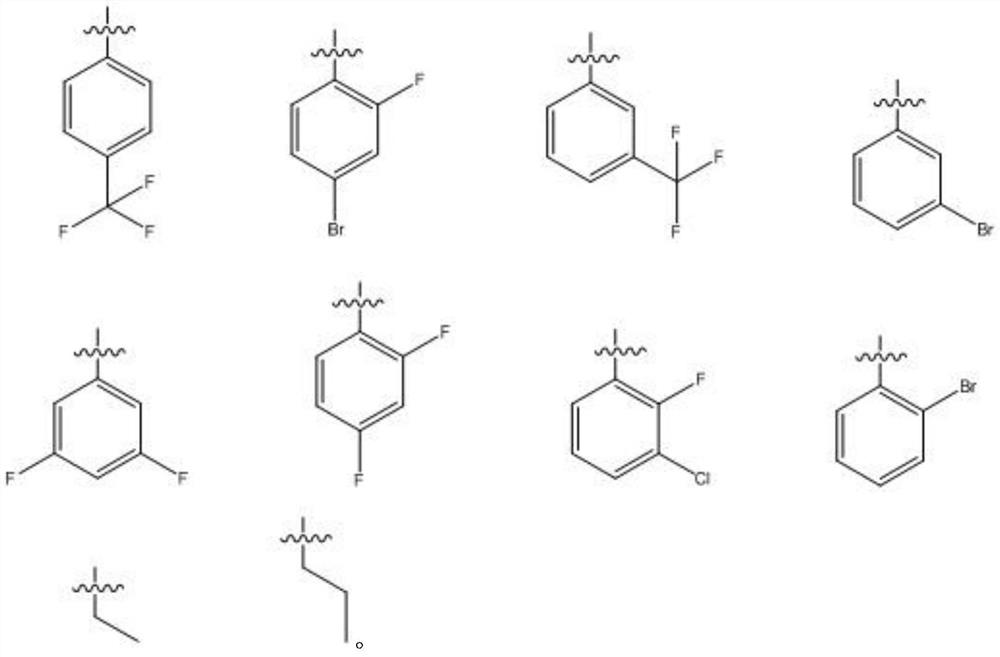
Preparation and antiviral application of baicalein derivative
A technology of baicalein and derivatives, applied in the directions of antiviral agents, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of poor hydrophilicity and low oral bioavailability, and achieve good enzyme inhibitory activity, preparation Simple and easy method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of GL01
[0044] Preparation of GL01
[0045] Accurately weigh 0.2206 g of baicalein with a purity of 98%, 0.8 mmol and 0.3053 g of 4-morpholinecarbonyl chloride with a purity of more than 98%, 2 mmol was placed in a 50 ml round-bottomed flask, 10 ml of anhydrous acetone was added to dissolve it, and then added 2 ml of pyridine. The above reaction solution was placed in an ice bath (0°C) and stirred for 1 h. The whole reaction process was under nitrogen protection. After monitoring the completion of the reaction by thin layer chromatography (TLC), add 20 ml of ice water to the reaction solution, let it stand for a while, and after the solid precipitates out, suction filtration, and the filter cake is washed with 20 ml of ethanol at 0°C. The residue was subjected to silica gel column chromatography (mobile phase: DCM), and purified by recrystallization to obtain a golden yellow powdery solid with a yield of 79%.
Embodiment 2
[0046] Example 2 Preparation of GL02
[0047] Preparation of GL02
[0048] Accurately weigh baicalein (purity: 98%) (0.2206g, 0.8mmol) and 4-chloro-3-nitrobenzenesulfonyl chloride (purity: 98%+) (0.5226g, 2mmol) into a 50ml round bottom flask , anhydrous acetone (10 ml) was added to dissolve it, followed by pyridine (2 ml). The above reaction solution was placed in an ice bath (0°C) and stirred for 1 h. The entire reaction process requires nitrogen protection. After monitoring the completion of the reaction by thin layer chromatography (TLC), add 20 ml of ice water to the reaction solution, let it stand for a while, and after the solid precipitates out, suction filtration, and the filter cake is washed with 20 ml of ethanol at 0°C. The residue was subjected to silica gel column chromatography (mobile phase: DCM), and purified by recrystallization to obtain an off-white powdery solid with a yield of 84%.
[0049] For the preparation method of GL03-GL31, refer to GL01 and GL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com