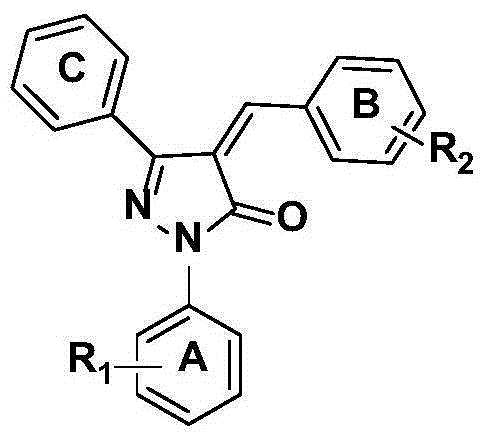
Substituted pyrazolone secretory aspartic protease inhibitor and method for preparing same
A pyrazolone, monosubstituted technology, applied in the field of medicine, can solve problems such as synthesis that have not yet been seen, and achieve the effect of good inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
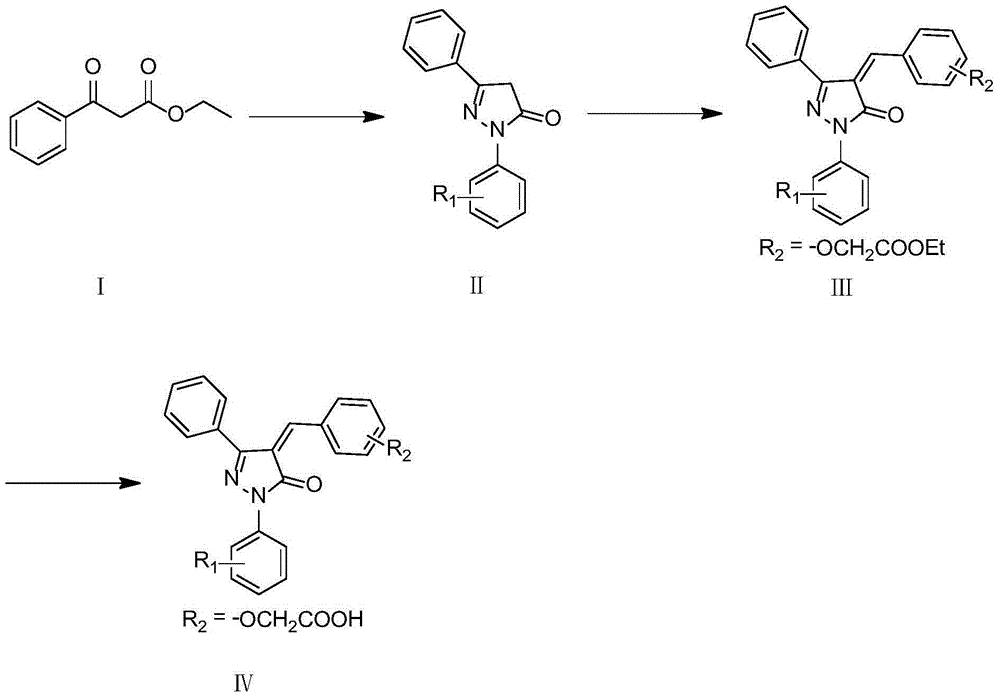
[0062] Example 1: 2,4-dihydro-2,5-diphenyl-3H-pyrazolin-3-one (Ⅱ, R 1 =H) Preparation
[0063] Weigh ethyl benzoylacetate (9.61g, 50mmol, 1.0equiv), phenylhydrazine hydrochloride (8.00g, 55mmol, 1.1equiv), acetic acid 2.0mL in a 100mL eggplant-shaped bottle, reflux the mixture for 3h, every hour board monitoring. After the reaction was completed, 20 mL of diethyl ether was added to the reaction solution, stirred in an ice bath for 30 min, the precipitate was filtered, washed with 30 mL of diethyl ether (a small amount of times), dried, and recrystallized from ethanol 3 times to obtain 5.1 g of a white solid, yield: 43% . 1 H-NMR (600MHz, DMSO-d 6 )δ: 7.98 (d, 1H, J = 8.26Hz), 7.85 (d, 2H, J = 7.60Hz), 7.50 (d, 2H, J = 7.73Hz), 7.45-7.47 (m, 1H), 7.36- 7.41(t, 2H, J=7.52Hz), 7.31-7.35(t, 2H, J=7.50Hz), 6.46(s, 1H), 3.87(s, 1H).ESI-MS(m / z): 237.43 [M+1].
Embodiment 2
[0064] Example 2: Ethyl 2-(2-{[5-oxo-1,3-diphenyl-1H-pyrazolidine-4(5H)-alkenylene]methyl}phenoxy)acetate (Ⅲ , R 1 = H, R 2 =2-OCH 2 Preparation of COOEt)
[0065] Weigh 2,4-dihydro-2,5-diphenyl-3H-pyrazolin-3-one (0.47g, 0.2mmol, 1.0equiv), ethyl 2-formylphenoxy acetate (0.49g , 0.24mmol, 1.2equiv) into a 25mL eggplant-shaped bottle, add 15mL of absolute ethanol to dissolve, then add 100μL of piperidine, stir and react at 60°C for 6h, a solid precipitates, filter the precipitate, recrystallize from ethanol, and obtain an orange solid 0.53g , Yield: 62%. 1 H-NMR (300MHz, DMSO-d 6 )δ:8.28(s,1),7.95(d,2H,J=7.76Hz),7.70-7.79(m,2H),7.54-7.66(m,4H),7.46(t,2H,J=7.70Hz ),7.07-7.31(m,4H),4.90(s,2H),4.11(dd,2H,J=7.10,14.24Hz),1.13(t,3H,J=7.15Hz).ESI-MS(m / z):427.41[M+1].
Embodiment 3
[0066] Example 3: 2-(2-{[5-oxo-1,3-diphenyl-1H-pyrazolidine-4(5H)-alkenylene]methyl}phenoxy)acetic acid (IV, R 1 = H, R 2 =2-OCH 2 COOH) preparation
[0067] Weigh 2-(2-{[5-oxo-1,3-diphenyl-1H-pyrazolidine-4(5H)-alkenylidene]methyl}phenoxy)ethyl acetate (0.2g, 0.47mmol, 1equiv) and LiOH·H 2 O (0.030g, 0.71mmol, 1.5equiv) in 5mL mixed solvent (THF:MeOH:H 2 O=3:2:1), stirred at room temperature for 0.5 h, evaporated the solvent under reduced pressure, added water (20 mL), adjusted the pH to 2.0-3.0 with 1M HCl, and precipitated. The precipitate was filtered, washed with cold water, and recrystallized from ethanol to obtain 0.12 g of an orange-red solid, yield: 64%, m.p.: 113-114°C. 1 H-NMR (300MHz, DMSO-d 6 )δ:13.09(brs,1H),7.85(d,2H,J=7.95Hz),7.73-7.55(m,2H),7.33-7.52(m,4H),7.20-7.30(m,5H),7.08 -7.18(m,2H),4.89(s,2H).ESI-MS(m / z):399.42[M+1].
[0068] All reagents used in the examples are commercially available analytically pure.
[0069] Compounds 2-25 in the table use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com