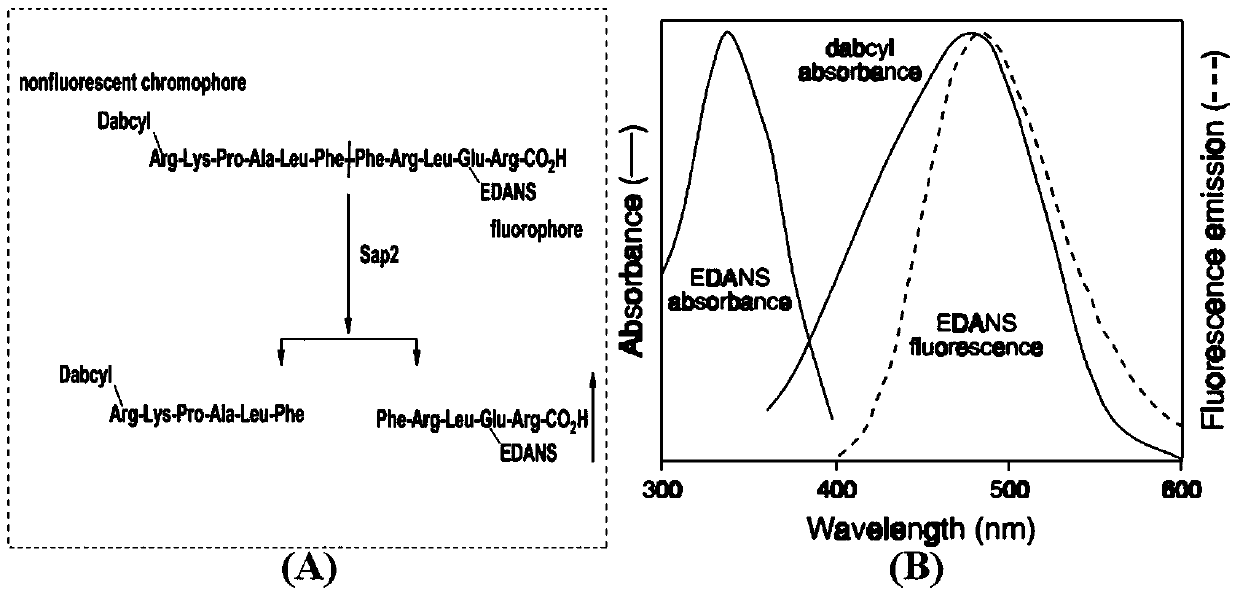
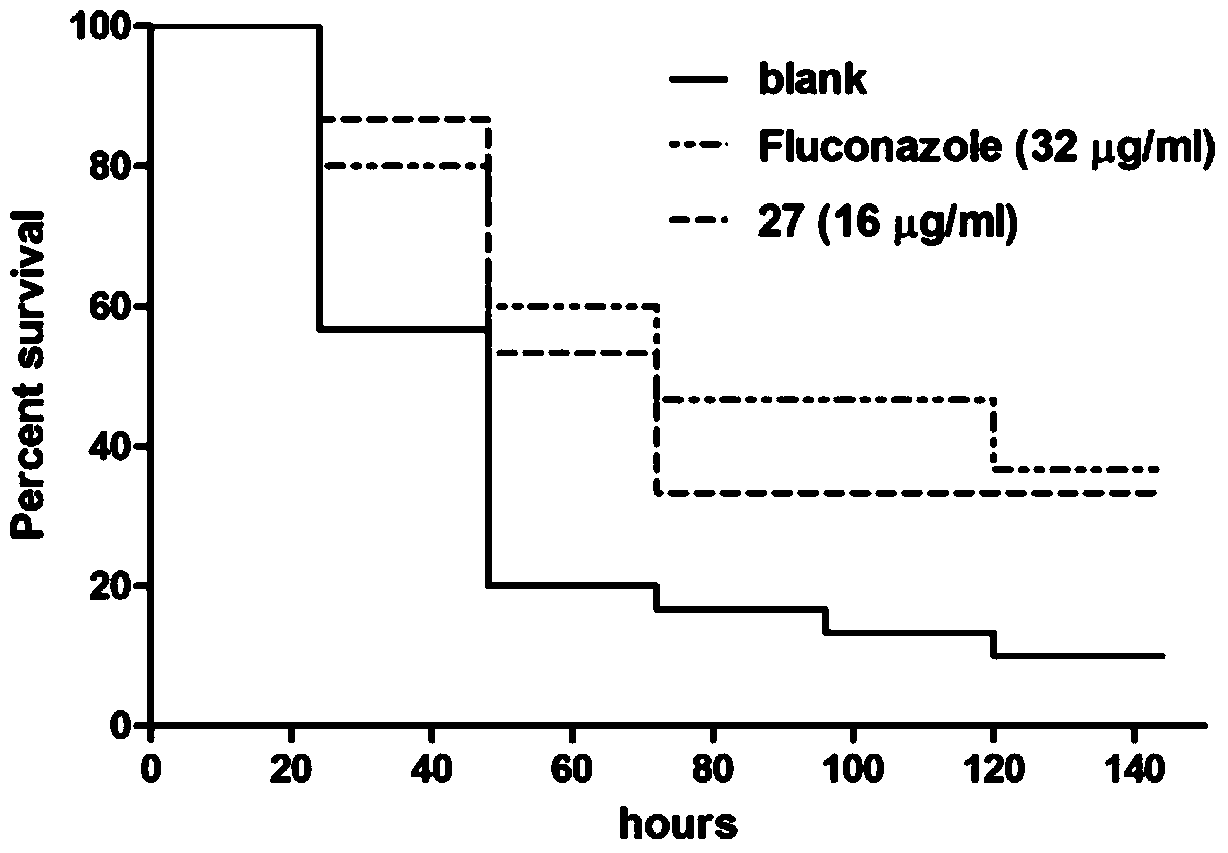
Substituted triaminotriazine secreting-type aspartic protease inhibitor and preparation method thereof
A technology of triazines and triamines, which is applied in the field of medicine, can solve the problems that are in the initial stage, and there is no such compound Sap2 enzyme inhibitory activity and antifungal activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1: Preparation of 4,6-dichloro-N-phenyl-1,3,5-triazine-2-amine (II, R 1 = ) preparation
[0132] Dissolve cyanuric chloride (9.22 g, 50 mmol, 1 equiv) in 30 mL of acetone, and stir in an ice bath to 0°C. Slowly add 20 mL of acetone solution dissolved in aniline (4.66 g, 50 mmol, 1 equiv) dropwise. Stirring was continued for 2 h under ice bath, followed by 2 h at room temperature. After the reaction was completed, 20 mL of ice water was added. After the solid was completely precipitated, it was filtered with suction to obtain 10.85 g of a white solid, yield: 90%. 1 H-NMR (300MHz, DMSO-d 6 )δ: 11.12 (s, 1H, NH), 7.69 (d, 2H, J = 2.36, 7.95Hz), 7.36 (d, 2H, J = 8.35Hz), 7.16 (t, 1H, J = 7.40Hz). ESI-MS(m / z):239.24[M-1].
Embodiment 2
[0133] Example 2: 6-Chloro-N 2 -(furan-2-ylmethyl)-N 4 -Phenyl-1,3,5-triazine-2,4-diamine (Ⅲ, R 1 = ) preparation
[0134] Dissolve 4,6-dichloro-N-phenyl-1,3,5-triazin-2-amine (2.41g, 10mmol, 1equiv) in 30mL tetrahydrofuran, add 2-furanmethylamine (0.92mL, 10mmol ,1equiv) and 5% Na 2 CO 3 The solution was 30 mL, stirred at room temperature for 12 hours, filtered to precipitate a solid, and washed 2-5 times with water to obtain 2.81 g of a white solid, yield: 93%. 1 H-NMR (300MHz, DMSO-d 6)δ:10.07(brs,1H,NH),8.59(brs,1H,NH),7.49-7.81(m,3H),7.19-7.38(m,2H),7.02(t,1H,J=7.18Hz) ,6.31-6.48(m,1H),6.15-6.30(m,1H),4.48(d,2H,J=5.05Hz).ESI-MS(m / z):302.49[M+1].
Embodiment 3
[0135] Example 3: N 2 -(furan-2-ylmethyl)-6-hydrazino-N 4 -Phenyl-1,3,5-triazine-2,4-diamine (IV, R 1 = ) preparation
[0136] 6-Chloro-N 2 -(furan-2-ylmethyl)-N 4 -Phenyl-1,3,5-triazine-2,4-diamine (1.51g, 5mmol, 1equiv) was dissolved in 50mL of dichloromethane, and hydrazine (0.64mL, 20mmol, 4equiv) was added to reflux and stirred for 12h. After the reaction, wash with 50 mL of water and 50 mL of saturated NaCl solution × 2, and dry over anhydrous sodium sulfate. The solution is added with 100 mL of petroleum ether, and the solid is filtered to obtain 0.99 g of a white solid, yield: 67%. 1 H-NMR (300MHz, DMSO-d 6 )δ:9.03(brs,1H,NH),7.92(brs,1H,NH),7.46-7.83(m,3H),7.24-7.44(brs,2H,NHNH 2 ),7.19(t,2H,J=7.92Hz),6.88(t,1H,J=7.07Hz),6.30-6.44(m,1H),6.12-6.29(m,1H),4.46(d,2H, J=5.21Hz),4.21(brs,1H,NHNH 2 ).ESI-MS(m / z):298.33[M+1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com