Chromone-containing benzimidazole bifurazan compound with Cy-FBP/SBPase inhibition effect and preparation method of chromone-containing benzimidazole bifurazan compound
A benzimidazole and inhibition technology, applied in the field of chromone-containing benzimidazole bifurazan compounds and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
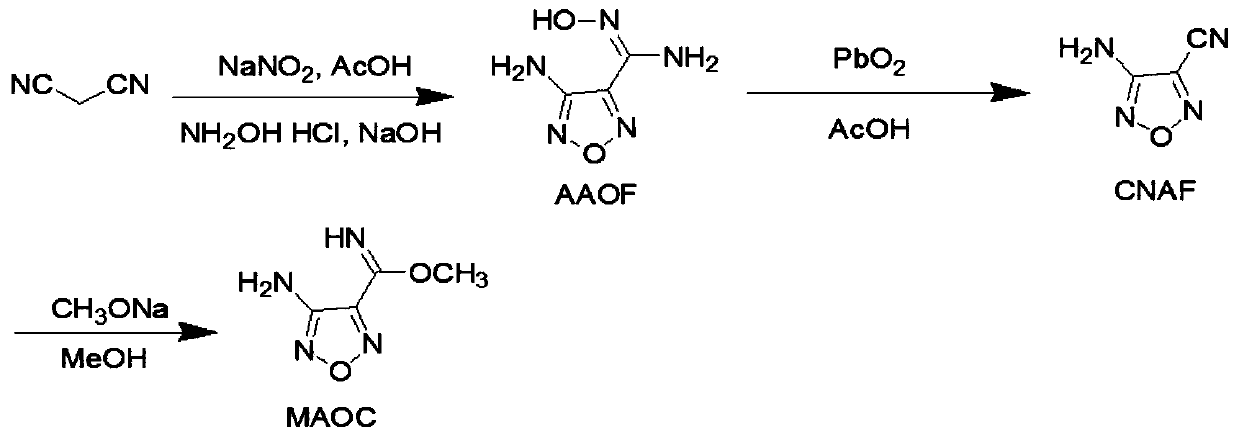
[0042] Intermediate MAOC
[0043] Preparation of 4-amino-1,2,5-oxadiazole-3-methoxyamidine (referred to as MAOC)
[0044]
[0045] Take 3.75g (57mmol) of malononitrile, 4.3g (62mmol) of sodium nitrite and 10mL of water and mix evenly and dissolve completely. Keeping at 0°C, slowly add 0.8mL of acetic acid dropwise, then stir at room temperature for 6 hours, the reaction solution turns from light yellow to orange red. Dissolve 9g (130mmol) of hydroxylamine hydrochloride in 14mL of water, and slowly add it dropwise, keeping the temperature below 20°C, a large amount of yellow foam is formed. The pH of the reaction solution was adjusted to 10 with 25% sodium hydroxide solution, and the reaction solution turned red and clear. Keep the reaction at 30°C for 10 hours, and then heat to reflux for 3 hours. After cooling, a yellow substance precipitated out. Suction filtration, washing, and drying gave 3.89 g (yield 45%) of light yellow powder 3-amino-4-amidoxime furoxan (AAOF). ...
Embodiment 2
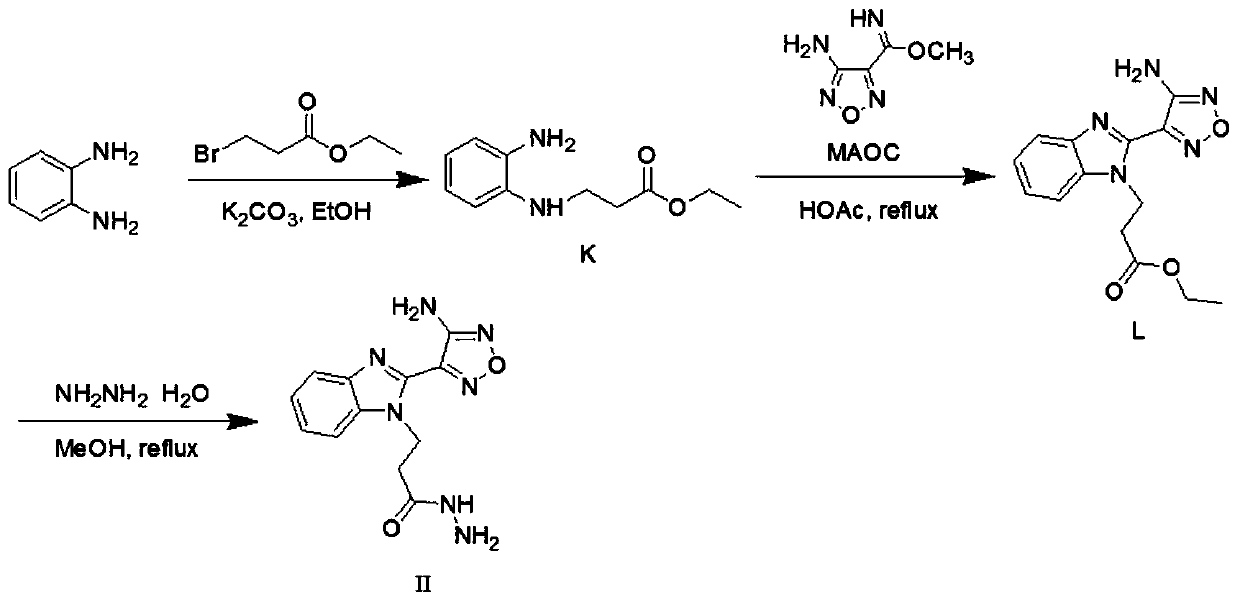
[0050] Intermediate II
[0051] Preparation of 3-(2-(4-amino-1,2,5-oxadiazole-3-)-1hydro-benzo[d]imidazole-1-)propionhydrazide (Intermediate II)
[0052]
[0053] Take 2.24g (20mmol) of o-phenylenediamine and dissolve it in 15mL of ethanol, add 1.43g (10mmol) of K 2 CO 3 , 3.76g (20mmol) ethyl bromopropionate, stirred at room temperature for 50 hours. Suction filtration, the filtrate was rotary evaporated to obtain a black mixture. With PE:EtOAc=1:1 column chromatography, one of the pure components was obtained as a red oily substance 3-((2-aminophenyl)amino) ethyl propionate (intermediate K) 0.88g (yield 20% ). 1 H NMR (400MHz, CDCl 3 )δ6.88-6.60 (m, 4H, ArH), 4.15 (q, J=7.2Hz, 2H, O-CH 2 ), 3.44(t, J=6.0Hz, 2H, N-CH 2 ), 2.66(t, J=6.0Hz, 2H, O=C-CH 2 ), 1.26(t, J=7.2Hz, 3H, CH 3 ).
[0054] Take 0.88g (4.2mmol) ethyl 3-((2-aminophenyl)amino)propionate (Intermediate K), 0.60g (4.2mmol) 4-amino-1,2,5-oxadiazole-3 - Place methoxyamidine (MAOC) in a round bottom fl...
Embodiment 3
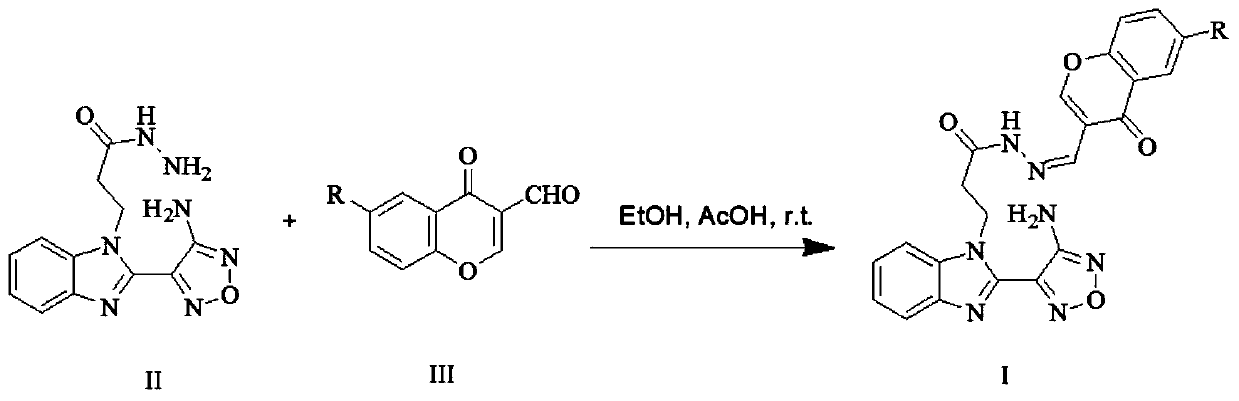
[0058] Compound I-1
[0059] Preparation of 3-[2-(4-aminofurazan-3-)-benzimidazole-1-]-propionic acid (4-oxo-4hydro-chromone-3-methylene)-hydrazide
[0060]
[0061] Take 287mg (1mmol) of Intermediate II and 1mmol of 3-formylchromone, mix and dissolve in 25ml of ethanol, add 3 drops of HOAc dropwise, and stir r.t. for 2h, a large amount of insoluble matter is formed. TLC monitors that the reaction is almost complete. Suction filtration, and wash with a large amount of ethanol several times, dry, obtain white powder, 3-[2-(4-aminofurazan-3-)-benzimidazole-1-]-propionic acid (4-oxo -4hydro-chromone-3-methylene)-hydrazide, 83% yield, m.p.218-220°C.
[0062] Molecular formula: C 22 h 17 N 7 o 4 ;
[0063] 1 H NMR (600MHz, DMSO-d 6 )δ11.60-11.31(m,1H,NH),8.81–7.21(m,10H,=CH-O,CH=N,ArH),7.12-6.80(m,2H,NH 2 ),5.13–4.78 (m,2H,CH 2 ),3.24-2.71(m,2H,CH 2 ); HR-MS (ESI): m / z=444.1356, calcd for C 22 h 17 N 7 o 4 [M+H] + :444.1342.
[0064] Compounds 2-11 were all pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com