Preparation method of voriconazole for injection
A technology for voriconazole and injection is applied in the field of preparation of voriconazole for injection, and can solve the problems of uneven stability of voriconazole-hydroxypropyl beta cyclodextrin inclusion complex, low effective components of voriconazole, instability of voriconazole in the presence of alkali, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
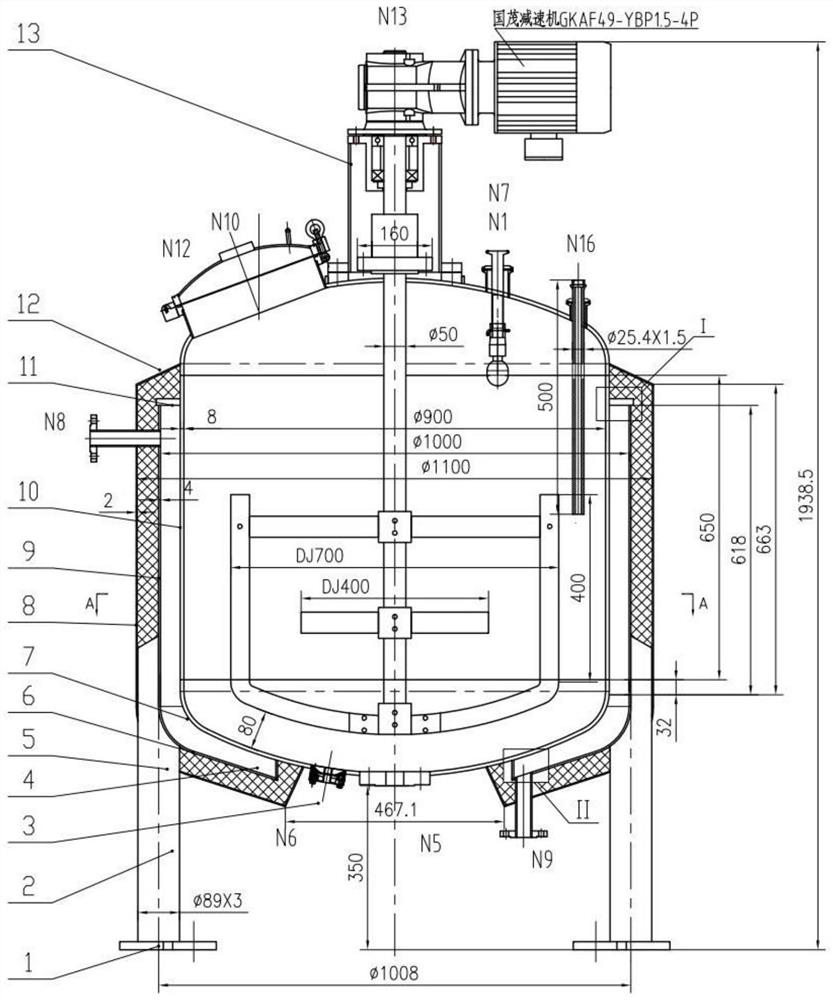
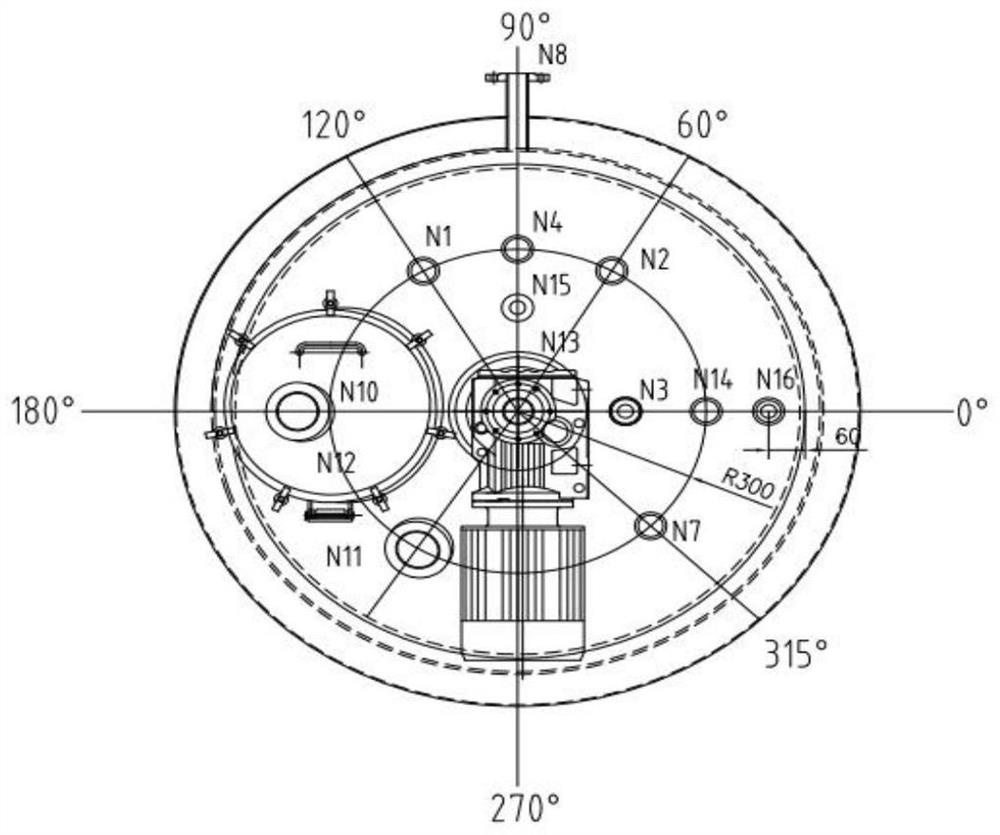
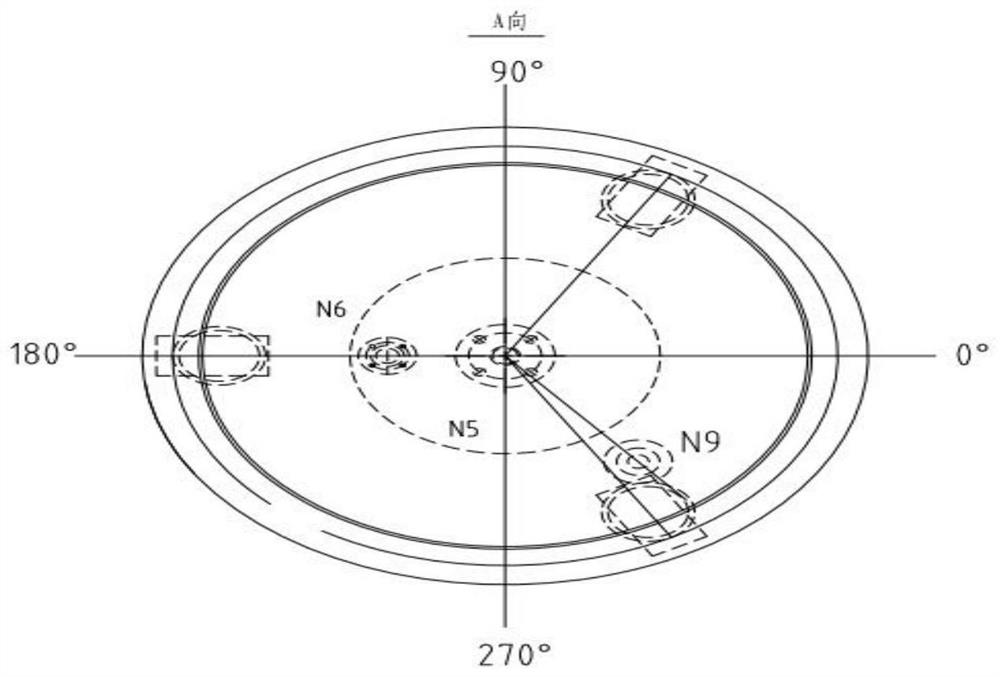
Image
Examples
preparation example Construction
[0048] The invention provides a preparation method of voriconazole for injection, comprising the following steps:
[0049] A preparation method of voriconazole for injection, comprising the following steps:
[0050] (1) Under the stirring condition of the stirring blade, hydroxypropyl beta-cyclodextrin is dissolved in water for injection to obtain an aqueous solution of hydroxypropyl-beta-cyclodextrin; the mass concentration of the aqueous solution of hydroxypropyl-beta-cyclodextrin 10.47-27.18%;
[0051] (2) under the stirring condition of stirring paddle, hydrochloric acid solution is mixed with voriconazole, obtains the hydrochloric acid solution of voriconazole;
[0052] (3) under the condition of stirring with a stirring blade, the hydrochloric acid solution of voriconazole is added to the aqueous solution of hydroxypropyl beta-cyclodextrin to obtain the voriconazole-hydroxypropyl-beta-cyclodextrin solution;
[0053](4) Under the condition of stirring with a stirring pa...
Embodiment 1
[0091] The prescribed amount of voriconazole for injection is as follows:
[0092]
[0093] The preparation method of voriconazole for injection comprises the following steps:
[0094] (1) Slowly add 800g of hydroxypropyl beta-cyclodextrin into 3300mL of water for injection with 80% of the prescription quantity, control the temperature between 50°C and 60°C, stir with a stirring paddle until the material is completely dissolved, and the stirring frequency is 30Hz .
[0095] (2) Add 200mL of 2mol / L hydrochloric acid solution to the prescribed amount of voriconazole, and stir while adding to completely dissolve the voriconazole, and the solution is clear.
[0096](3) Slowly add the completely dissolved voriconazole-hydrochloric acid solution into the dissolved hydroxypropyl beta-cyclodextrin aqueous solution, between 50°C and 60°C, stir with a stirring paddle at a stirring frequency of 30Hz, and complete the addition Stirring was then continued for 30 minutes.
[0097] (4)...
Embodiment 2
[0101] The amount of raw materials and the preparation method are the same as those in Example 1, except that the batches are different. The obtained voriconazole product for injection is recorded as batch 20190102.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com