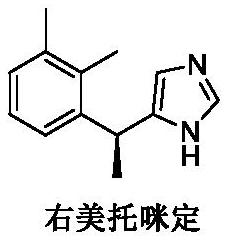
Preparation method of dexmedetomidine hydrochloride degradation impurity
A technology for dexmedetomidine hydrochloride and impurities, which is applied in the field of preparation of dexmedetomidine hydrochloride to degrade impurities, and can solve the problems of short preparation steps and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
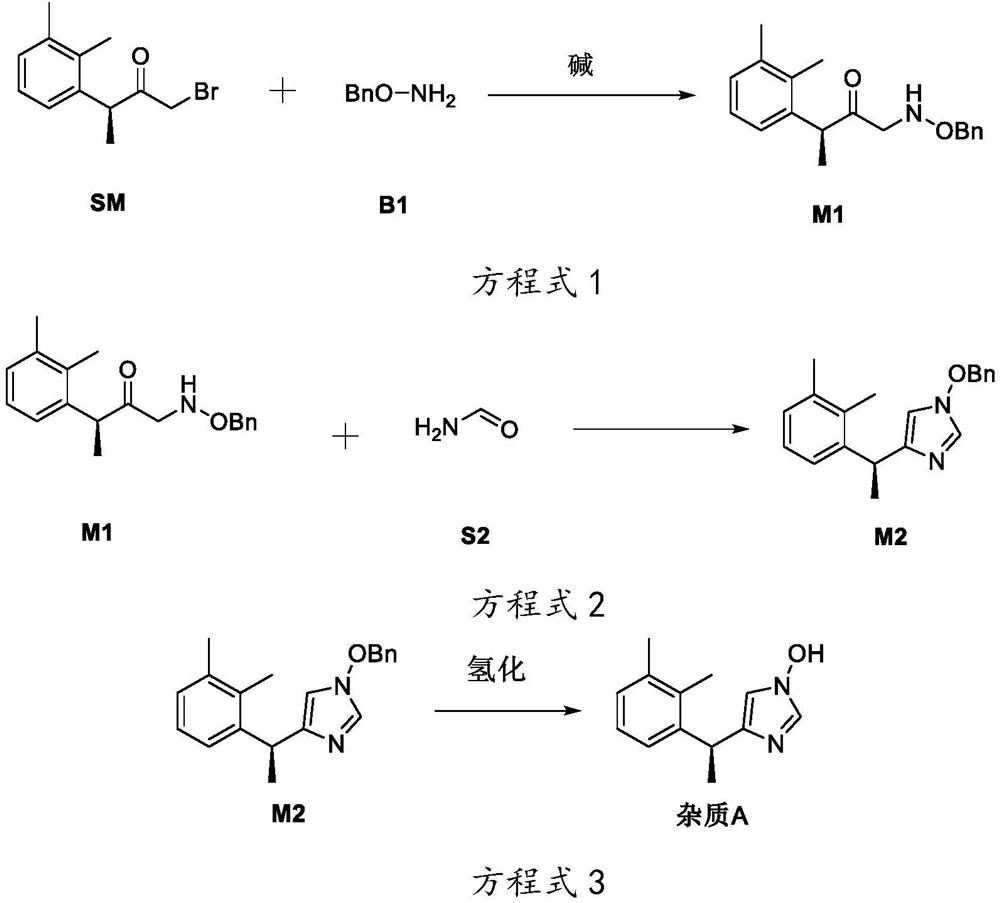
[0052] This embodiment provides a method for preparing dexmedetomidine hydrochloride degradation impurities, which is used to prepare impurity A in the degradation impurities of dexmedetomidine hydrochloride, comprising the following steps:
[0053] Compound M1 was prepared as shown in Equation 1:
[0054]
[0055] The preparation of compound M1 is specifically as follows according to equation 1:
[0056] Add 1.00 g of (S)-1-bromo-3-(2,3-dimethylphenyl) butan-2-one and 20 mL of N,N-dimethylformamide into a 50 mL reaction flask, and add Add 1.53g of cesium carbonate and 0.58g of O-benzyl hydroxylamine successively in the reaction flask, stir the reaction at room temperature, thin layer chromatography shows that (S)-1-bromo-3-(2,3-dimethylphenyl ) butan-2-one reaction disappears to obtain a reaction solution; under stirring, pour 60mL of water into the reaction solution, extract 3 times with 20mL of ethyl acetate, combine the organic layers obtained, and use 30mL of saturate...
Embodiment 2
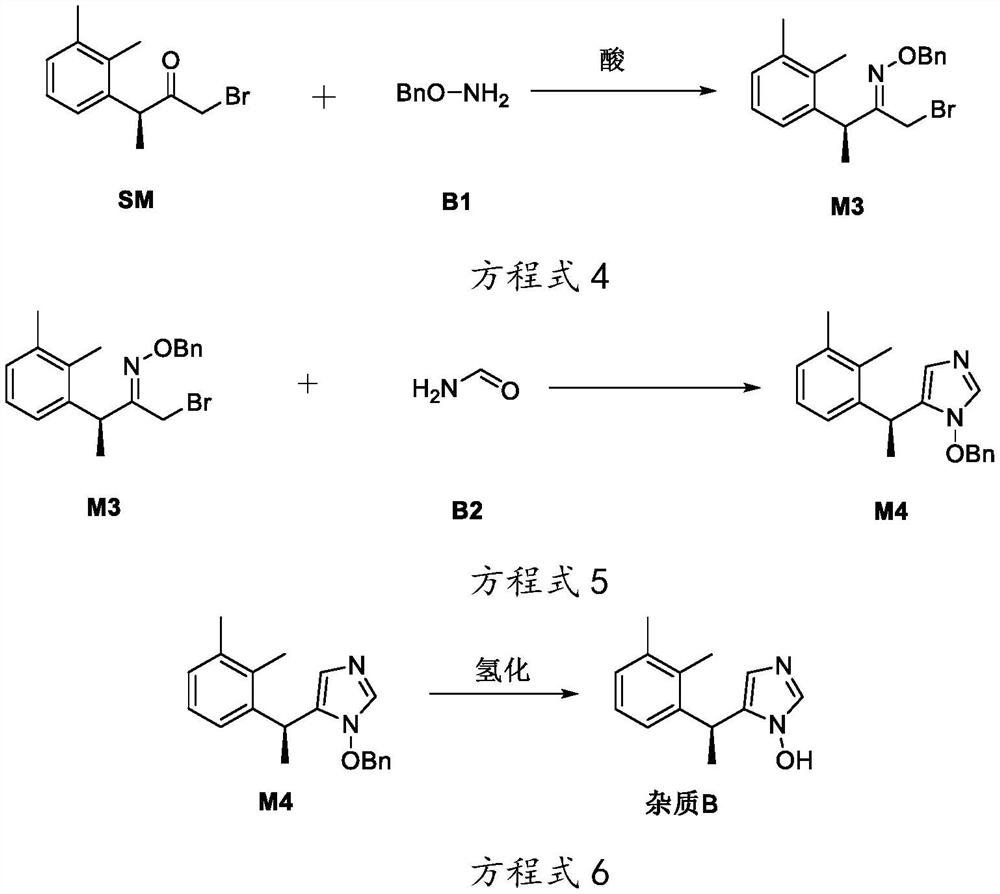
[0069] This embodiment provides a preparation method for degrading impurities by dexmedetomidine hydrochloride, which is used to prepare impurity B in the impurities degraded by dexmedetomidine hydrochloride, comprising the following steps:
[0070] The preparation of compound M3 is shown in equation 4:
[0071]
[0072] The preparation of compound M3 is specifically as follows according to equation 4:
[0073] Add 2.00g of (S)-1-bromo-3-(2,3-dimethylphenyl)butan-2-one and 18mL of dichloromethane into a 50mL reaction flask, and sequentially add 2mL of acetic acid and 1.10g of O-benzylhydroxylamine were stirred at room temperature for 5h, and thin-layer chromatography showed (S)-1-bromo-3-(2,3-dimethylphenyl)butan-2-one disappear, add 50mL of water to the reaction bottle, stir and stand for liquid separation, the separated water layer is extracted 3 times with 20mL dichloromethane, combined to obtain the organic layer, and the organic layer is washed once with 30mL saturate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com