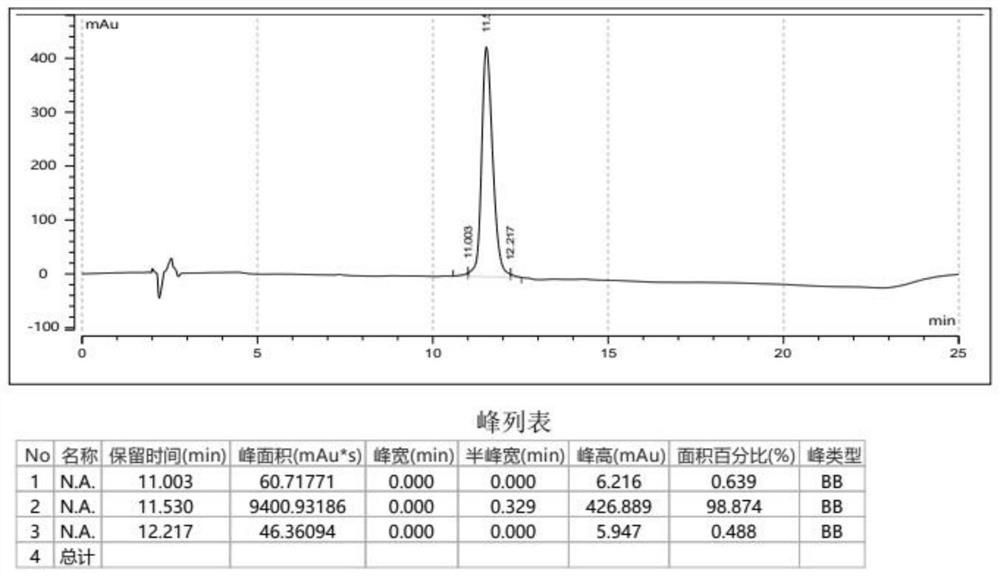
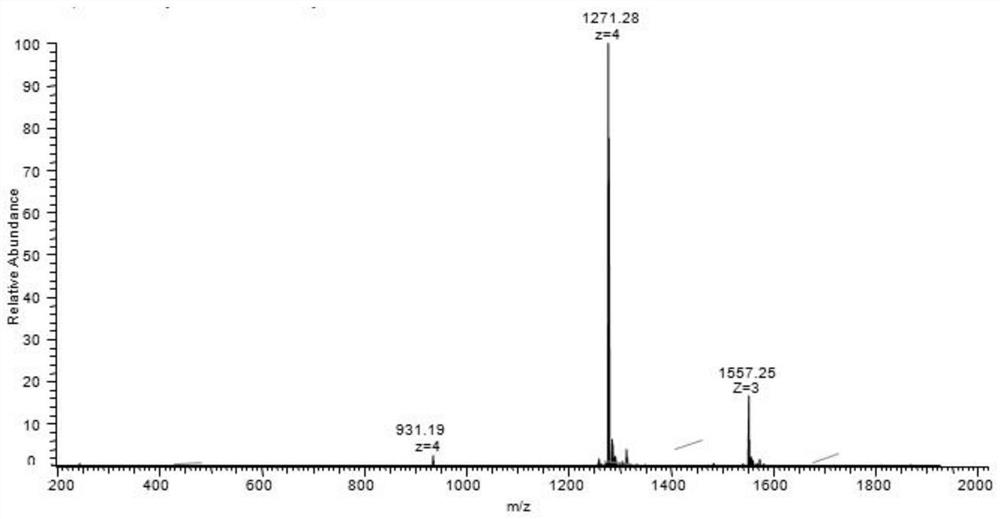
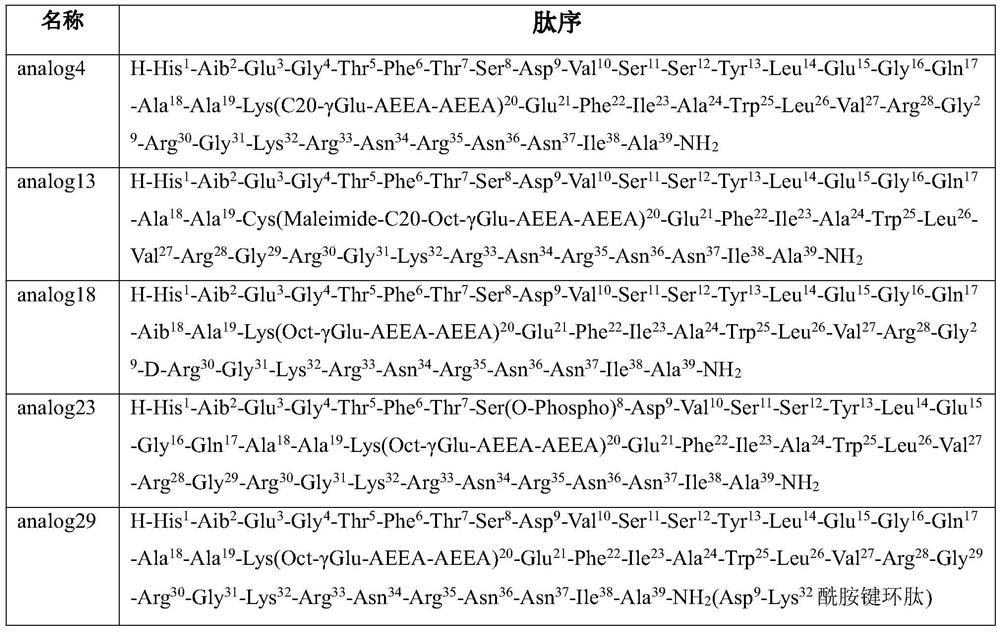
GLP-1R/GCGR/GIPR triple receptor agonist and application thereof
A parent, ac-his technology, applied in the GLP-1R/GCGR/GIPR triple receptor agonist and its application field, can solve problems such as increasing satiety, raising blood sugar and accelerating insulin resistance, achieving good hypoglycemic and promoting Effects of islet secretion, improvement of tolerance, and reduction of degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1: Preparation of linear peptide resin for analog1-analog9
[0124] Weigh 62.5 g (20 mmol) of Fmoc-Rink-Amide resin (Sub=0.32 mmol / g) into the reaction column, wash with DMF for 3 times, and then swell with DMF for 30 minutes. The Fmoc protecting group was then removed with DBLK, followed by 6 washes with DMF. Weigh 18.66 g (60 mmol) of Fmoc-Ala-OH and 8.91 g (66 mmol) of HOBt, dissolve them in DMF, add 11.35 g of DIC (90 mmol) in an ice-water bath at 0°C, activate for 5 minutes, add to the reaction column, and react for 2 hours , and then remove the Fmoc protecting group with DBLK. Repeat the above operation, and couple Fmoc-Ile-OH, Fmoc-Asn(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Lys(Boc)-OH, Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Fmoc- Val-OH, Fmoc-Leu-OH, Fmoc-Trp(Boc)-OH, Fmoc-Ala-OH, Fmoc-Ile-OH, Fmoc-Phe-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Lys( Alloc)-OH or Fmoc-Dap(Alloc)-...
Embodiment 2
[0125] Example 2: Removal of the side chain protecting group Alloc of analog1-analog9
[0126] The fully protected peptide resin obtained in Example 1 was washed 3 times with DCM. Weigh 43.2 grams of phenylsilane, measure 500ml of DCM, add to the reaction column, after reacting for 5 minutes, add 11.55 grams of Pd 0(Ph 3 P) 4 , reacted for 1 hour. Then the resin was washed 3 times with DCM, the peptide resin was washed with DMF solution for 30 minutes, the resin was washed with DMF for 3 times, and then the resin was washed with DCM for 3 times to obtain a peptide resin from which Alloc was selectively removed, which was set aside.
[0127] The resulting resin was divided into 10 fractions, 2 mmol each, for the synthesis of analog1 to analog9.
Embodiment 3
[0128] Example 3: Coupling of analog1 side chains
[0129] Weigh 2.31 g (6 mmol) of Fmoc-AEEA-OH, 0.89 g (6.6 mmol) of HOBt, dissolve in DMF, add 1.13 g of DIC (9 mmol) in an ice-water bath at 0°C, activate for 5 minutes, and add the Peptide resin, reacted for 2 hours, and then removed the Fmoc protecting group with DBLK. Repeat the above operation, and couple Fmoc-AEEA-OH, Fmoc-Glu-OtBu, and tert-butyl octadecanedioate in sequence; after the reaction, wash with DMF for 6 times and DCM for 3 times, then add methanol Wash 3 x 10 minutes, and vacuum dry to obtain 18.8 g of analog1 peptide resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com