Dextromethorphan quinidine orally disintegrating tablet and application thereof
A technology for dextromethorphan and orally disintegrating tablets, applied in the field of orally disintegrating tablets containing dextromethorphan and quinidine and its preparation, can solve the problems of taste masking of the undisclosed dextromethorphan quinidine composition, and achieve disintegration The effects of short solution time, improved medication experience, and easy-to-accept taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
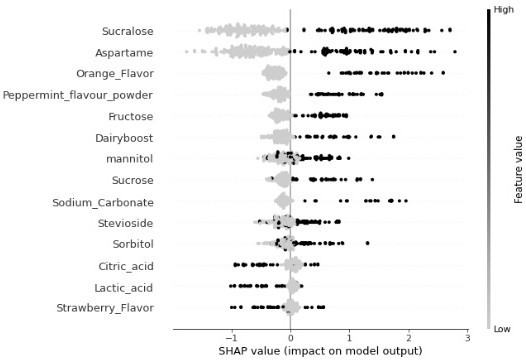
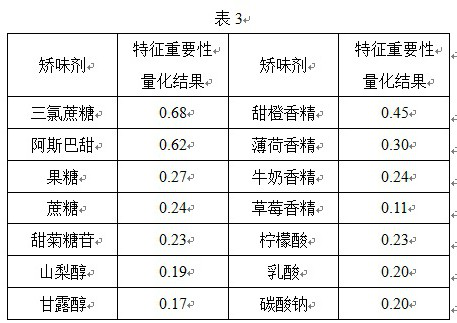
Image
Examples
Embodiment 1
[0050] Weigh 20mg dextromethorphan hydrobromide, 10mg quinidine sulfate and 2mg colloidal silicon dioxide, mix and sieve, add 32mg mannitol, 16mg xylitol, 40mg microcrystalline cellulose, 10mg crospovidone , 10mg croscarmellose sodium, 4mg sucralose and 4mg peppermint essence, mixed and sieved, mixed evenly again, added 2mg sieved sodium stearyl fumarate, mixed evenly and used dry granulation mechanism grain. Then add 10 mg of sieved crospovidone, 10 mg of sieved croscarmellose sodium and 2 mg of sieved sodium stearyl fumarate, mix well and then compress into tablets to obtain dextromethorphanqui Nitin orally disintegrating tablets.
Embodiment 2
[0052] Weigh 20mg dextromethorphan hydrobromide, 10mg quinidine sulfate and 2mg colloidal silicon dioxide, mix and sieve, add 32mg mannitol, 16mg xylitol, 40mg microcrystalline cellulose, 20mg crospovidone , 4 mg of sucralose and 4 mg of peppermint essence, mixed and sieved, mixed evenly again, adding 2 mg of sieved sodium stearyl fumarate, mixed evenly, and then granulated using a dry granulator. Add 20 mg of sieved crospovidone and 2 mg of sieved sodium stearyl fumarate, mix evenly, and press into tablets to obtain dextromethorphan quinidine orally disintegrating tablets.
Embodiment 3
[0054] Weigh 20mg dextromethorphan hydrobromide, 10mg quinidine sulfate and 2mg colloidal silicon dioxide, mix and sieve, add 32mg mannitol, 16mg xylitol, 40mg microcrystalline cellulose, 20mg crospovidone , 4 mg of aspartame and 4 mg of mint essence, mixed and sieved, mixed again evenly, added 2 mg of sieved sodium stearyl fumarate, mixed evenly, and then granulated using a dry granulator. Add 20 mg of sieved crospovidone and 2 mg of sieved sodium stearyl fumarate, mix evenly, and press into tablets to obtain dextromethorphan quinidine orally disintegrating tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com