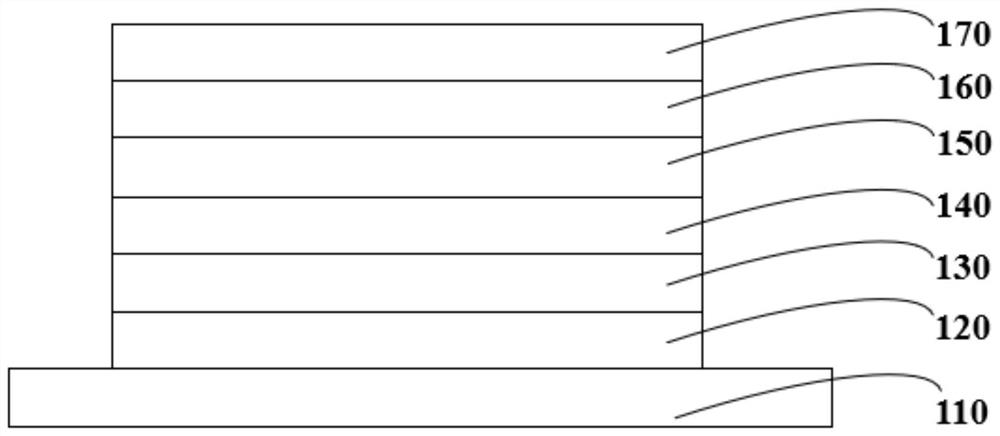
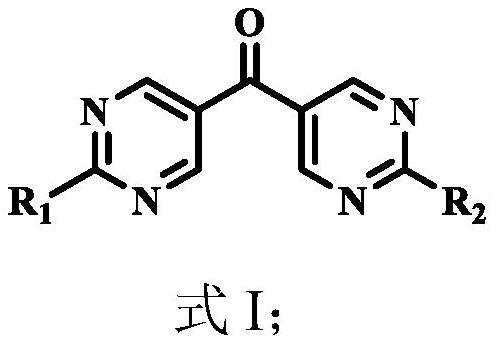
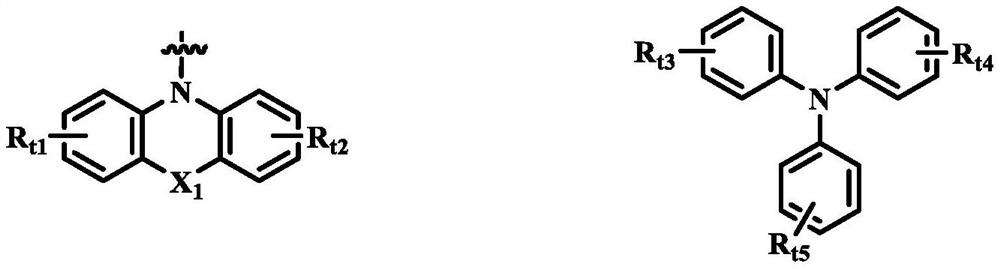Organic compound, organic light-emitting display panel and application thereof
An organic compound, an unsubstituted technology, applied in the field of organic electroluminescent materials, can solve the problems of blue phosphorescent materials instability, high cost, environmental pollution, waste of triplet excitons, etc., to suppress non-radiative transitions and improve Effect of narrowing the half peak width and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] This embodiment provides an organic compound M1, the structural formula of which is as follows:
[0082]
[0083] The preparation method of this organic compound comprises the following steps:
[0084] (1) Preparation of compound c-1
[0085]
[0086] At -78°C, add n-BuLi (2.20mL, 5.1mmol) to a solution of 2,5-dibromopyrimidine (a-1) (1.18g, 5mmol) in THF (20mL), and stir at -78°C 1 h, then a solution of 2-bromopyrimidine-5-carboxaldehyde (b-1) (0.95 g, 5.1 mmol) in THF (20 mL) was added dropwise, and stirred at -78°C for another 2 h. The mixture was quenched with aqueous hydrochloric acid at 0 °C and partitioned between water and DCM. The organic layer was washed with water, washed with Na 2 SO 4 Dry; add pyrimidinium chlorochromate (2.20 g, 10 mmol) to the DCM layer, and stir at room temperature for 4 h. The reaction mixture was filtered and washed with DCM. After evaporation of the solvent, purification by column chromatography gave compound c-1 (0.84 g, ...
Embodiment 2
[0095] This embodiment provides an organic compound M2, the structural formula is as follows:
[0096]
[0097] The preparation method of this organic compound comprises the following steps:
[0098] (1) Synthesis of compound d-2:
[0099]
[0100] Take 2-bromo-N-phenylaniline (e-1) (2.48g, 10mmol) in dry tetrahydrofuran (20mL) and add n-BuLi (1.6M hexane, 12.80mL, 20mmol) dropwise at -78°C. After stirring for 1 h, a solution of fluorenone (f-1) (1.98 g, 11 mmol) was added while stirring. After the reaction mixture was heated to room temperature and stirred for 3 h, it was quenched with a small amount of water, and the resulting mixture was concentrated and diluted with chloroform (50 mL). The solution was washed with water and extracted twice with chloroform. The mixture was dried over anhydrous sodium sulfate and concentrated to obtain the hydroxyl intermediate. The hydroxyl intermediate was refluxed with chloroform (40mL) and methanesulfonic acid (0.96g, 10mmol) f...
Embodiment 3
[0109] This embodiment provides an organic compound M3, the structural formula of which is as follows:
[0110]
[0111] The preparation method of this organic compound comprises the following steps:
[0112] (1) Synthesis of compound d-3:
[0113]
[0114] Take 2-bromo-N-phenylaniline (e-1) (2.48g, 10mmol) in ultra-dry tetrahydrofuran (20mL) solution, and drop n-BuLi (1.6M hexane, 12.80mL, 20mmol) at -78°C add. After stirring for 1 h, a solution of xanthone (f-2) (2.16 g, 11 mmol) was added while stirring. After the reaction mixture was heated to room temperature and stirred for 3 h, it was quenched with a small amount of water, and the resulting mixture was concentrated and diluted with chloroform (50 ml). The solution was washed with water and extracted twice with chloroform. The mixture was dried over anhydrous sodium sulfate and concentrated to obtain the hydroxyl intermediate. The hydroxyl intermediate was refluxed with chloroform (40mL) and methanesulfonic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com