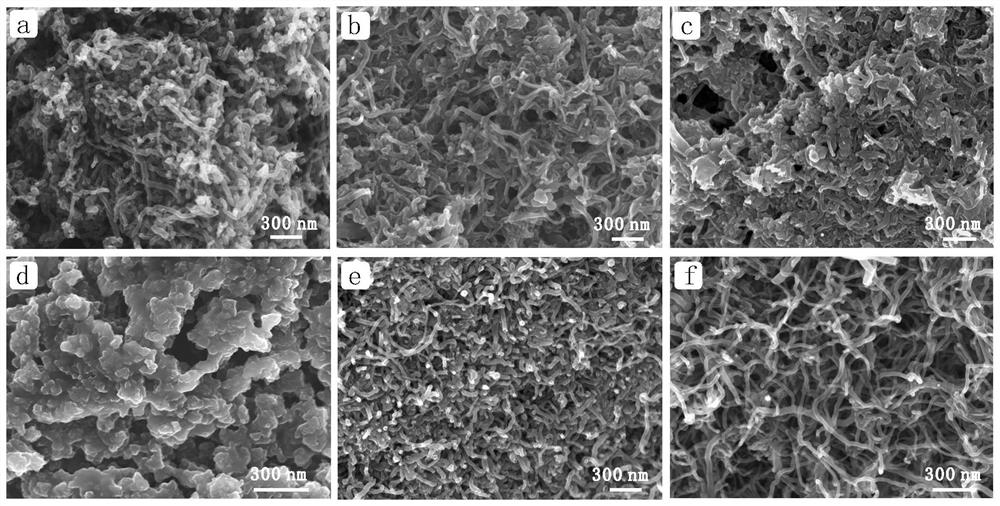
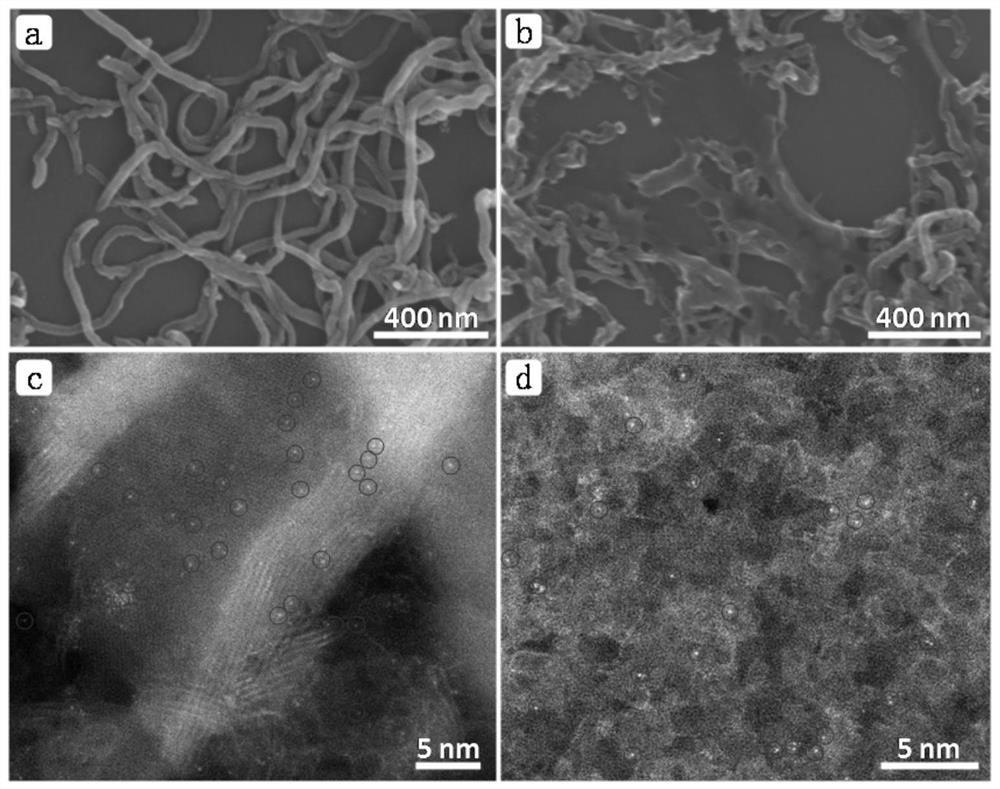
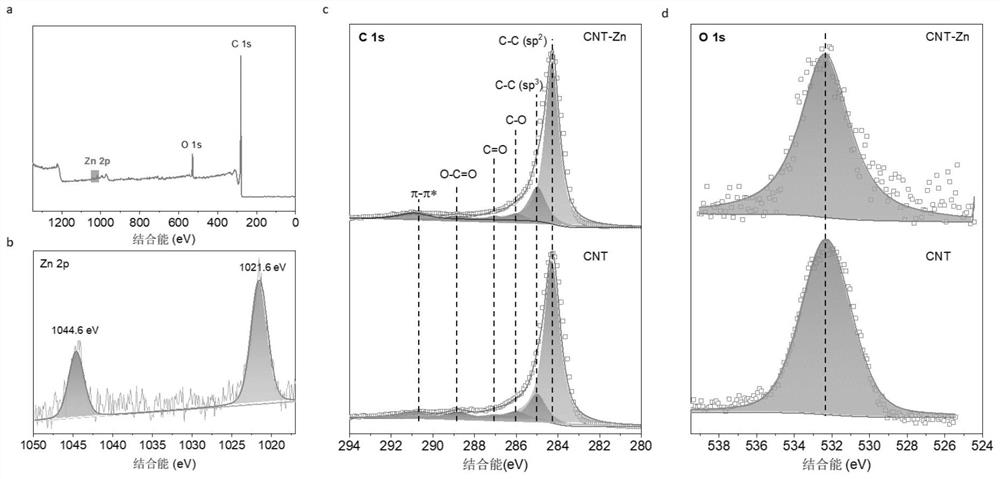
Preparation method of CNT-Zn monatomic catalytic material
A technology of catalytic materials and atoms, applied in electrolysis components, electrodes, electrolysis process, etc., can solve the problems of unclear relationship between active sites and catalytic performance, cumbersome preparation methods, and high cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The CNT-Zn composite was prepared according to the following steps:
[0023] 1. Disperse the carbon nanotubes in a mixed solution of deionized water and ethanol (volume ratio 1:1), ultrasonically crush for 1 hour, then filter, and disperse the carbon nanotubes in 2M H 2 SO 4 In the solution, ozone gas was passed through for 1 hour under the condition of a water bath at 25°C.
[0024] 2. Ball mill 2.2g of potassium chloride, 2.2g of sodium chloride and 4.4g of zinc chloride, and then add 640mg of carboxylated carbon nanotubes for ball milling.
[0025] 3. Put the above black powder in a tube furnace, raise the temperature to 800°C at a rate of 5°C / min under an argon atmosphere, keep the temperature constant for 2 hours, and then lower it to room temperature at a rate of 5°C / min to obtain CNT-Zn- 800 single-atom materials.
[0026] 4. Disperse the prepared CNT-Zn-800 single-atom material in 1M HCl solution, and stir at 80° C. for 12 hours. After filtration, it was vac...
Embodiment 2
[0028] 1. Disperse the carbon nanotubes in a mixed solution of deionized water and ethanol (volume ratio 1:1), ultrasonically crush for 1 hour, then filter, and disperse the carbon nanotubes in 2M H 2 SO 4 In the solution, ozone gas was passed through for 1 hour under the condition of a water bath at 25°C.
[0029] 2. Ball mill 2.2g of potassium chloride, 2.2g of sodium chloride and 4.4g of zinc chloride, and then add 640mg of carboxylated carbon nanotubes for ball milling.
[0030] 3. Put the above-mentioned black powder in a tube furnace, raise the temperature to 900°C at a rate of 5°C / min under an argon atmosphere, keep the temperature constant for 2 hours, and then lower it to room temperature at a rate of 5°C / min to obtain CNT-Zn- 900 single-atom materials.
[0031] 4. Disperse the prepared CNT-Zn-900 single-atom material in 1M HCl solution, and stir at 80°C for 12 hours. After filtration, it was vacuum-dried at 60°C.
Embodiment 3
[0033] 1. Disperse the carbon nanotubes in a mixed solution of deionized water and ethanol (volume ratio 1:1), ultrasonically crush for 1 hour, then filter, and disperse the carbon nanotubes in 2M H 2 SO 4 In the solution, ozone gas was passed through for 1 hour under the condition of a water bath at 25°C.
[0034] 2. Ball mill 2.2g of potassium chloride, 2.2g of sodium chloride and 4.4g of zinc chloride, and then add 640mg of carboxylated carbon nanotubes for ball milling.
[0035] 3. Put the above black powder in a tube furnace, raise the temperature to 1000°C at a rate of 5°C / min under an argon atmosphere, keep the temperature constant for 2 hours, and then lower it to room temperature at a rate of 5°C / min to obtain CNT-Zn- 1000 monatomic materials.
[0036] 4. Disperse the prepared CNT-Zn-1000 single-atom material in 1M HCl solution, and stir at 80°C for 12 hours. After filtration, it was vacuum-dried at 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com