Antibodies against CAIX with reduced affinity for neonatal Fc receptor
An affinity, antibody technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, preparations for in vivo experiments, etc., can solve problems such as patient exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0560] Example 1: Antibodies for Binding to CAIX
[0561] overview
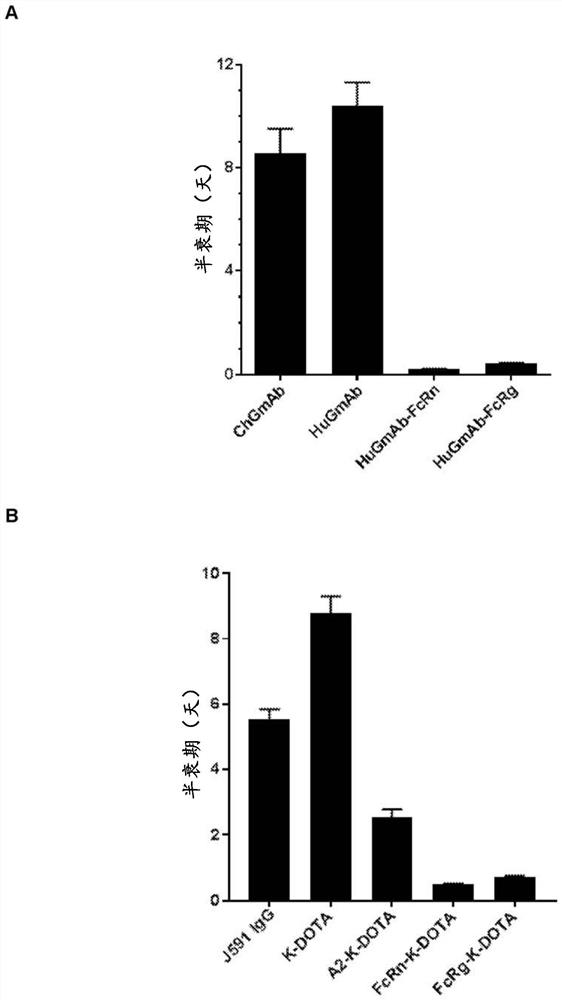
[0562] Humanized antibody variable region genes were cloned into vectors encoding unmodified human IgG1 heavy chain constant domain and human kappa light chain constant domain. The chimeric antibody was additionally cloned into a vector encoding a modified human IgG1 heavy chain constant domain containing mutations H310A and H435Q that abolish FcRn and protein A binding (Andersen et al., 2012), or mutation S228P (to stabilize the hinge (Angal et al., 1993)) and L235E (removing effector function (Reddy et al., 2000)) as well as modified human IgG4 heavy chain constructs of the FcRn ablation mutations described above. Chimeric and humanized antibodies were transiently expressed in HEK EBNA cells and tested for binding to human carbonic anhydrase IX (CAIX) using Biacore single-cycle kinetic analysis. Selected lead antibodies were purified by protein A or protein G and analyzed by analytical SEC, competition ...
example 2
[0601] Example 2: Antibodies for Binding to PSMA
[0602] overview
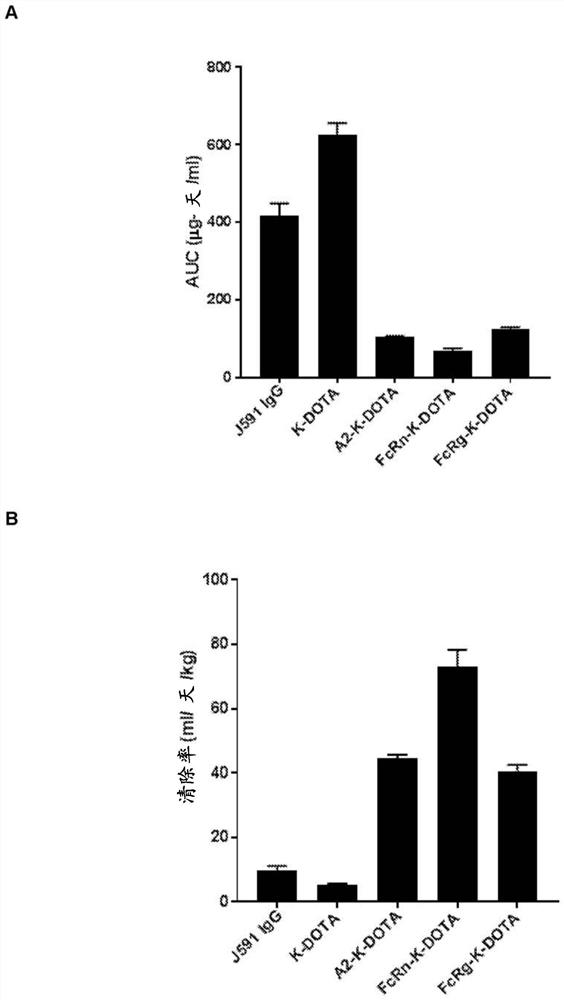
[0603] The antibody VH gene sequences of two antibodies (ANT4044 and ANT4044-A2) were cloned into three different human IgG double expression vectors encoding unmodified IgG1 containing mutations H310A and H435Q (eliminating FcRn binding and protein A binding ( Andersen et al., 2012)) IgG1 (termed IgG1(H310A, H435Q)), and with the same FcRn depletion mutation as above, as well as the hinge stabilizing S228P mutation (Angal et al., 1993) and the Fc silent L235E mutation (Reddy et al., 2000 ) modified IgG4 (referred to as IgG4 (S228P, L235E, H310A, H435Q)). Each dual expression vector also contained the antibody VK gene sequence common to both ANT4044 and ANT4044-A2.
[0604] A total of five antibodies were transiently transfected and expressed in CHO cells and purified using protein A (ANT4044-A2 IgG1) or protein G (ANT4044 and ANT4044-A2 both expressed as IgG1 (H310A, H435Q) and IgG4 (S228P , L235E, H310...
example 3
[0638] Example 3: Antibody Conjugation
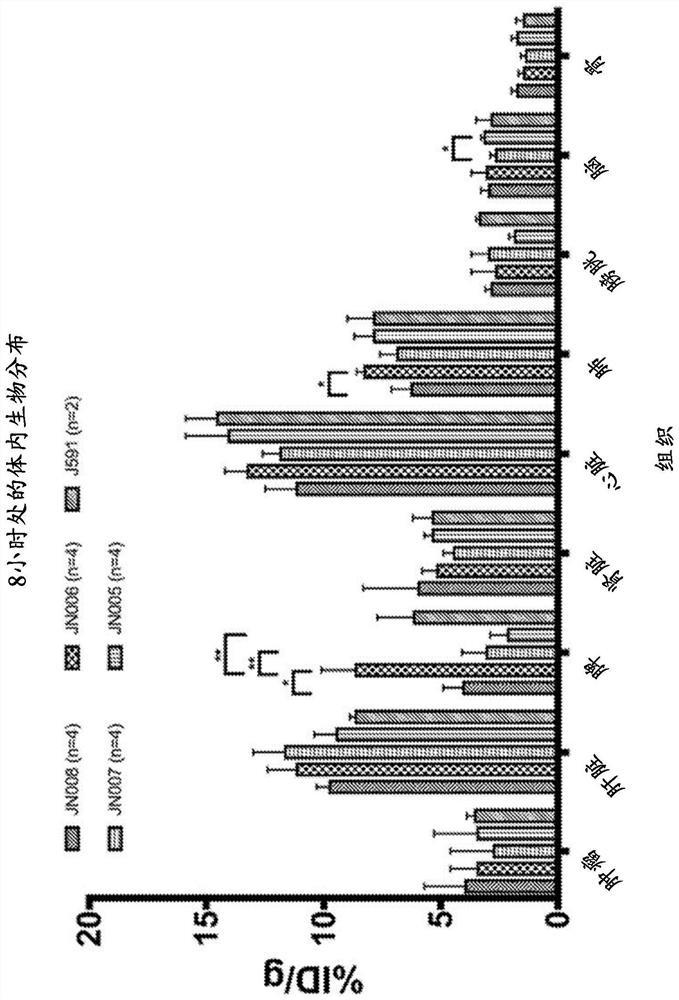
[0639] Antibodies ANT4044-IgG1, ANT4044-A2-IgG1, ANT4044-IgG1 H310A H435Q (also known as ANT4044-IgG1-2M) and ANT4044-IgG4 S228P L235E H310A H435Q (also known as ANT4044-IgG4-4M) with ThioBridge TM - PEG(6u)-DOTA reagent or NHS-DOTA reagent conjugation.
[0640] Thio Bridge TM is a PolyTherics proprietary disulfide-conjugated linker and is described in
[0641] DOTA is the chelator payload, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid monoamide.
[0642] Thio Bridge TM DOTA Conjugation Evaluation: ANT4044-IgG1 was prepared as a 6-10 mg / mL solution in reaction buffer (20 mM sodium phosphate, pH 7.5, 150 mM NaCl, 20 mM ethylenediaminetetraacetic acid (EDTA)). To ANT4044-IgG1 in reaction buffer (6-10 mg / mL, 40°C) was added 6-10 equivalents per antibody of tris(2-carboxyethyl)phosphine (TCEP) or DTT 10 mM. Adjust the antibody concentration to 5 mg / mL by diluting with reaction buffer. The reducing mixture was incubated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com