Application of umbilical artery in preparation of graft blood vessel material for coronary artery bypass grafting and method for preparing graft blood vessel material for coronary artery bypass grafting
A technology of coronary artery bypass and umbilical artery, applied in the field of biomedicine, to prevent spasm, less impact on physical properties of blood vessels, and long-term preservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Physiological and anatomical research of embodiment 1 umbilical artery
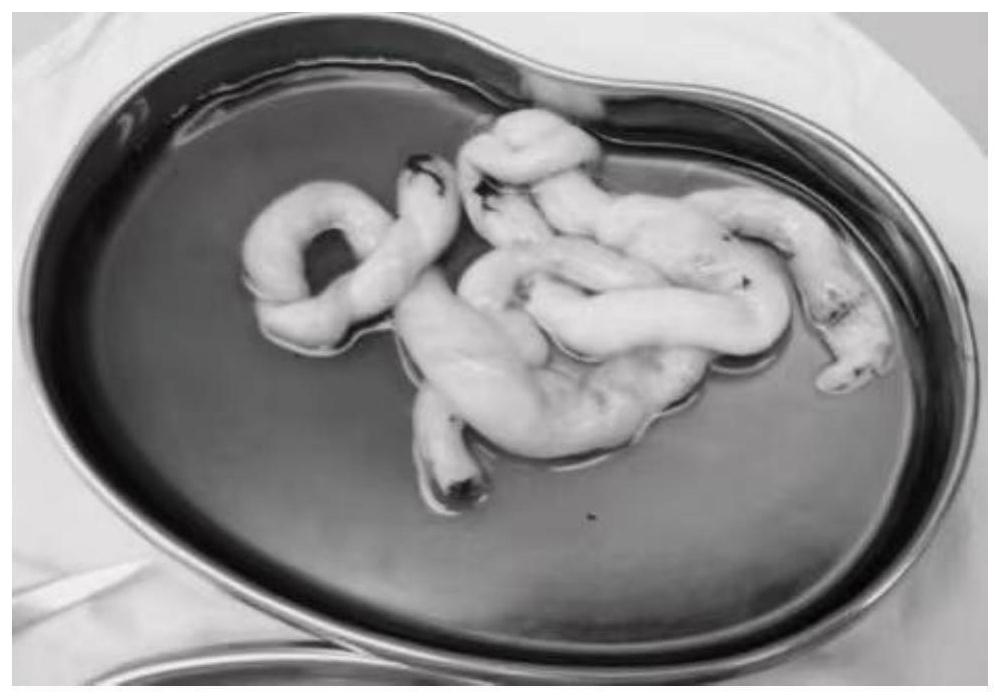
[0079] 1. Collection and preparation of umbilical artery materials. The collection of umbilical arteries was completed within 4-6 hours after fetal delivery, and all operations were performed under sterile conditions. Such as figure 1 shown.
[0080] Obtain blood vessels from the umbilical cord strictly following non-invasive techniques; after obtaining the umbilical cord, place it in sterile saline containing papaverine and 1‰ heparin, and use it to flush the vessel lumen to prevent vasospasm and thrombosis; keep the prepared solution at room temperature for 37 ℃; perfusion pressure <200mmHg. After fully freeing the umbilical artery, place it in normal saline with papaverine (30mg / 100ml) and 1‰ heparin.
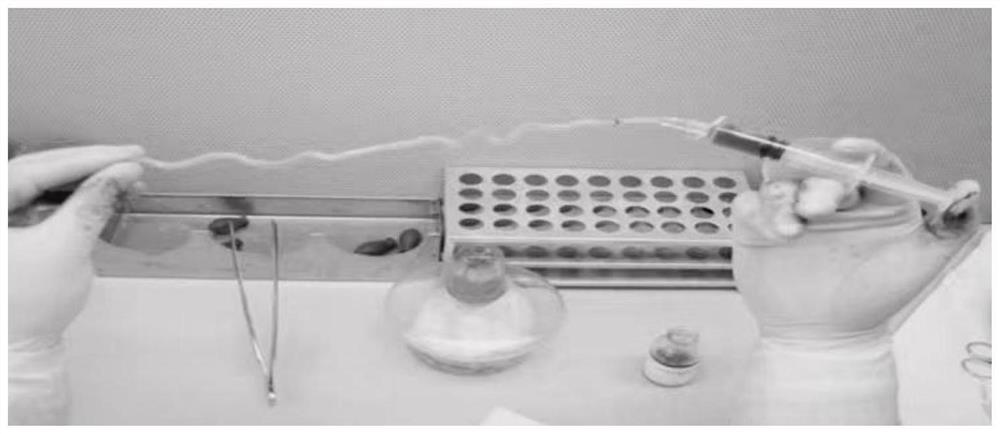
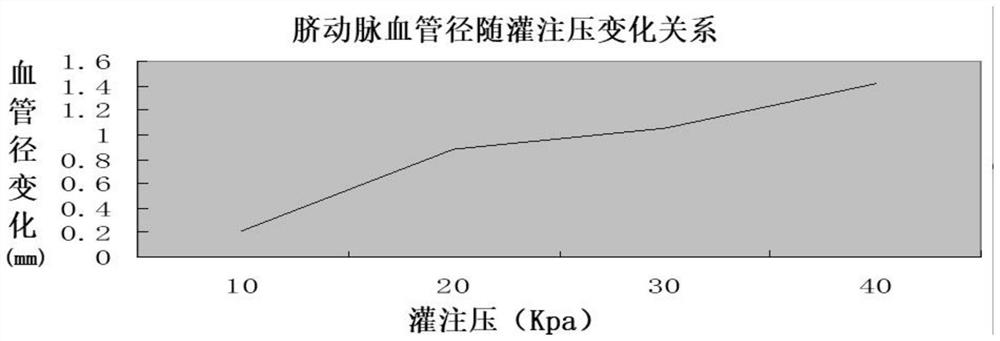
[0081] 2. Detection of the physical properties of the umbilical artery--the compliance and elasticity of the umbilical artery and the pressure test.
[0082] Experimental method: Measure the...
Embodiment 2
[0121] Example 2 Antigen removal, anti-calcification treatment and related performance detection of umbilical artery after treatment
[0122] Purpose:
[0123] It can be known from Example 1 that the umbilical artery of newborns has the potential to be used as a material for coronary artery bypass grafting. However, if the neonatal umbilical artery is used clinically, it is an allogeneic blood vessel and has antigenicity. Therefore, it must be treated with anti-antigen and anti-calcification before using the umbilical artery. In this experiment, we will use the anti-antigenic and anti-calcification methods we have explored to treat the umbilical artery, and then test the effect of this method and the impact on the related properties of the umbilical artery, hoping to find a clear effect, little impact on the physical properties of the blood vessel, and safe , Simple and easy method, suitable for possible clinical use in the future.
[0124] Materials and Methods
[0125] 1....
Embodiment 3
[0213] The animal experiment research of embodiment 3 umbilical artery application
[0214] Purpose:
[0215] It can be known from Example 2 that after cryopreservation in liquid nitrogen + glutaraldehyde treatment of the umbilical artery, the anti-antigen and anti-calcification effects are good, and the physical properties of the blood vessels are less affected, and it is easy to implement, so it becomes our alternative for animal experiments. Vascular material. In order to further explore the applicability of the umbilical artery, we designed an animal model to conduct an in vivo study to observe the vascular patency and vascular morphological changes of the umbilical artery treated with cryogenic liquid nitrogen + glutaraldehyde, which provides a basis for the clinical application of the umbilical artery. Basic experimental data.
[0216] Materials and Methods
[0217] 1. Main materials and instruments:
[0218]
[0219] 2. Method:
[0220] (1) Rabbit carotid artery...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com