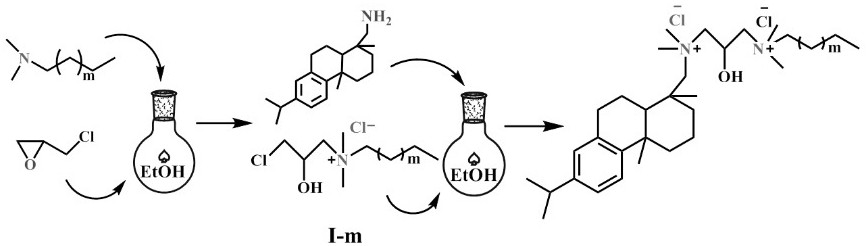
Asymmetric Gemini cationic surfactant and preparation method thereof
A surfactant and cationic technology, which is applied in the field of asymmetric Gemini cationic surfactant and its preparation, can solve problems such as difficult to find faults, and achieve the effects of easy industrial production, simple preparation process, and improved emulsification performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Dissolve 10.67 g of N,N-dimethyldodecylamine (0.05 mol) in 100 mL of ethanol. To the solution was added 2.19 mL of hydrochloric acid (0.06 mol) and stirred at 25 °C for 10 min. 11.57 mL of epichlorohydrin (0.125 mol) was added dropwise to the flask and stirred at 60 °C for 2 h. The solvent and residual epichlorohydrin were removed by vacuum rotary evaporation. Then recrystallize from acetone to obtain intermediate I-12;
[0024] (2) 10.24 g of intermediate I-12 (0.03 mol) and 8.81 g of dehydroabietylamine (0.031 mol) were dissolved in 100 mL of ethanol and poured into a single-necked flask equipped with a condenser. The mixture was stirred at 90 °C for 24 h. Then the solvent was removed by vacuum rotary evaporation, and the product obtained after recrystallization, filtration and vacuum drying was the asymmetric Gemini cationic surfactant RGS-2-12.
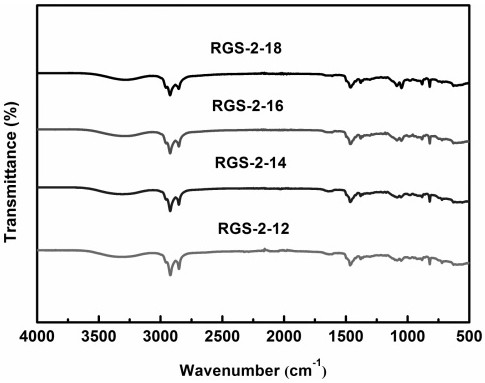
[0025] The infrared spectrum of the asymmetric Gemini cationic surfactant RGS-2-12 under the conditions of this e...
Embodiment 2
[0028] (1) Dissolve 12.07 g of N,N-dimethyltetradecylamine (0.05 mol) in 100 mL of ethanol. To the solution was added 2.19 mL of hydrochloric acid (0.06 mol) and stirred at 25 °C for 10 min. 11.57 mL of epichlorohydrin (0.125 mol) was added dropwise to the flask and stirred at 60 °C for 2 h. The solvent and residual epichlorohydrin were removed by vacuum rotary evaporation. Recrystallization from acetone yields intermediate I-14;
[0029] (2) 11.08 g of intermediate I-14 (0.03 mol) and 8.81 g of dehydroabietylamine (0.031 mol) were dissolved in 110 mL of ethanol and poured into a single-necked flask equipped with a condenser. The mixture was stirred at 90 °C for 24 h. Then, the solvent was removed by vacuum rotary evaporation, and the product obtained after recrystallization, filtration and vacuum drying was the asymmetric Gemini cationic surfactant RGS-2-14.
[0030] The infrared spectrum of the quaternary ammonium salt rosin-based Gemini surfactant RGS-2-14 under the con...
Embodiment 3
[0033](1) Dissolve 13.48 g of N,N-dimethylhexadecylamine (0.05 mol) in 100 mL of ethanol. To the solution was added 2.19 mL of hydrochloric acid (0.06 mol) and stirred at 25 °C for 10 min. 11.57 mL of epichlorohydrin (0.125 mol) was added dropwise to the flask and stirred at 60 °C for 2 h. The solvent and residual epichlorohydrin were removed by vacuum rotary evaporation. Recrystallization from acetone yields intermediate I-16;
[0034] (2) 11.91 g of intermediate I-16 (0.03 mol) and 8.81 g of dehydroabietylamine (0.031 mol) were dissolved in 120 mL of ethanol and poured into a single-necked flask equipped with a condenser. The mixture was stirred at 90 °C for 24 h. Then the solvent was removed by vacuum rotary evaporation, and the product obtained after recrystallization, filtration and vacuum drying was the asymmetric Gemini cationic surfactant RGS-2-16.
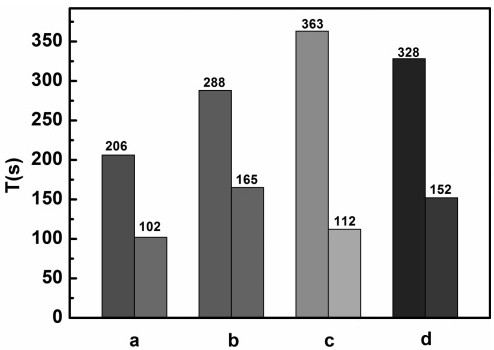
[0035] The emulsification performance diagram of the asymmetric Gemini cationic surfactant RGS-2-16 in this example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com