Pharmaceutical composition for rhinitis and preparation method thereof
A composition and drug technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of long taking cycle, heavy dose burden of rhinitis population, slow onset of effect, etc., to achieve fast onset of effect and shorten treatment time, effect of dose reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
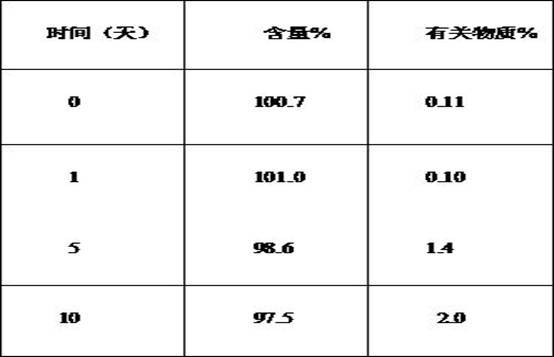
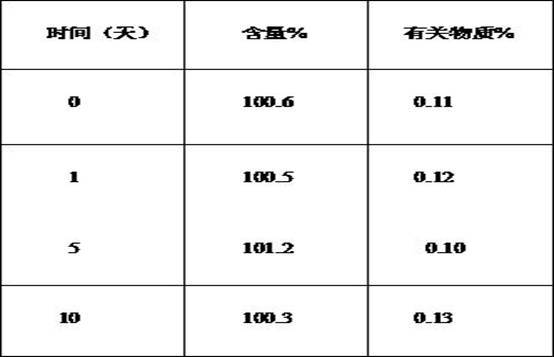
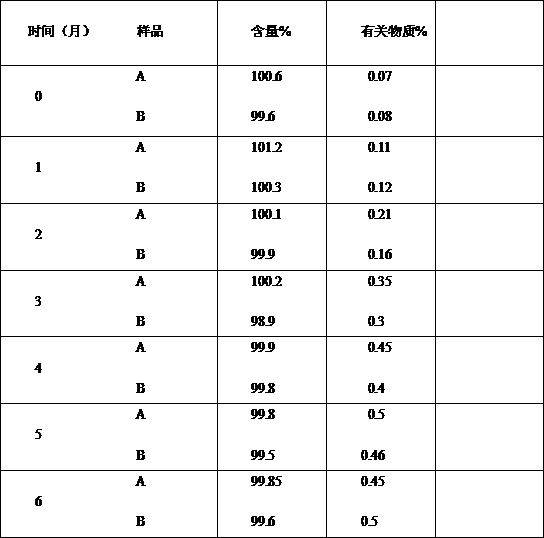
Image
Examples
Embodiment 1
[0025] First, 300 mg of roxithromycin raw material was made into micropowder A for standby use, 9000 mg of microcrystalline cellulose and 1000 mg of sodium carboxymethyl cellulose were dissolved in 300 ml of purified water for high-speed homogenization to obtain B for standby use, and 6 ml of glycerol was dissolved in 200 ml of purified water. Stir well to obtain solution 1; add 10 g of glucose and 5 mg of disodium EDTA to 100 ml of purified water and dissolve to obtain solution 2; add solution 1 to solution 2 with stirring, add A and B respectively to homogenize, and make up the purified water to 1000 ml. quality, adjust the pH to 7.0, and homogenize into 100 cans.
Embodiment 2
[0026] Embodiment 2, first make 200mg of roxithromycin raw material into micropowder A for standby use, dissolve 6000mg microcrystalline cellulose and 700mg sodium carboxymethyl cellulose in 200ml purified water, and obtain B by high-speed homogenization, and dissolve 50ml glycerol in 600ml In purified water, stir well to obtain solution 1; add 5 mg of disodium EDTA and 15 g of glucose into 100 ml of purified water, and dissolve to obtain solution 2; stir solution 1 into solution 2, add A and B respectively to homogenize, and make up for purified water To 1000ml, homogenize, adjust pH to 7.1, and homogenize into 100 cans.
Embodiment 3
[0027] Embodiment 3, first make roxithromycin raw material 400mg into micropowder A for standby use, dissolve 2000mg microcrystalline cellulose and 2400mg sodium carboxymethyl cellulose in 200ml purified water to obtain B by high-speed homogenization, and dissolve 10ml glycerol in 200ml In purified water, stir well to obtain solution 1; add 0.1 mg of disodium EDTA and 10 g of glucose into 200 ml of purified water, and dissolve to obtain solution 2; stir solution 1 into solution 2, add A and B respectively and homogenize to supplement the purification Water to 1000ml, homogenize, adjust pH to 6.9, and homogenize into 100 cans.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com