Lurasidone hydrochloride pharmaceutical composition and preparation method thereof
A technology of lurasidone hydrochloride and a composition is applied in the field of lurasidone hydrochloride pharmaceutical composition and preparation thereof, and can solve the problems of poor stability of nanosuspension, difficult to store for a long time, etc. The preparation process is simple and the effect of reducing the food effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
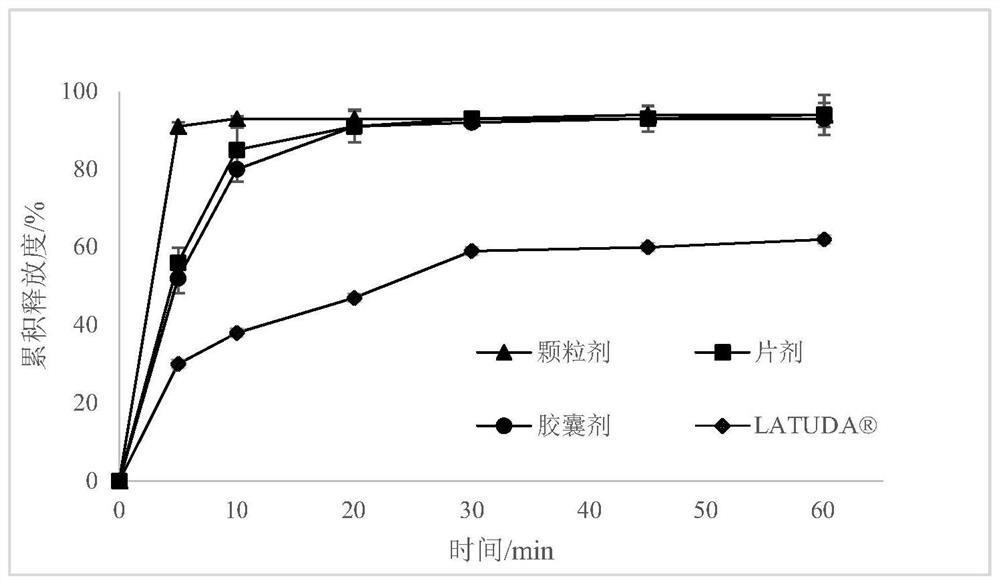
[0133] Preparation of nanocrystal suspension: dissolve stabilizer hypromellose E5 and Tween 80 in water to prepare a stabilizer aqueous solution, add lurasidone hydrochloride, and stir well; the obtained lurasidone hydrochloride is suspended The liquid was added to the ball mill, and 0.3 mm zirconia beads were added, the peristaltic pump was turned on at 44 rpm, the grinding chamber speed was 10 m / s, and the lurasidone hydrochloride nanocrystal suspension was obtained by wet grinding for 4 h.
[0134]
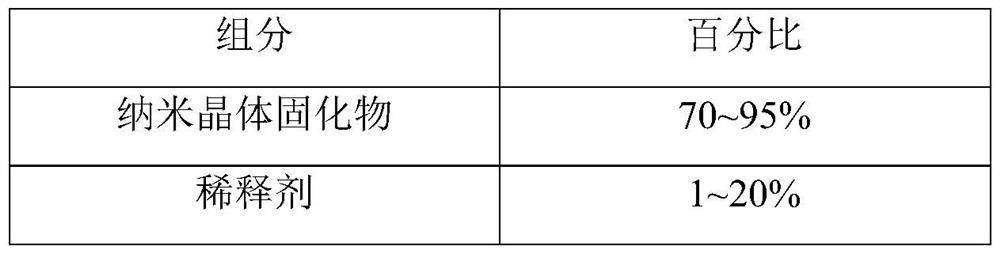
[0135] Preparation of nanocrystal solidified product: take the lurasidone hydrochloride nanocrystal suspension obtained in the above steps, filter through a 300-mesh sieve, add redispersant sucrose and stir well, use filler lactose as a carrier, and filter through a fluidized bed Granulation to obtain a solidified product of lurasidone hydrochloride nanocrystals.
[0136]
[0137]
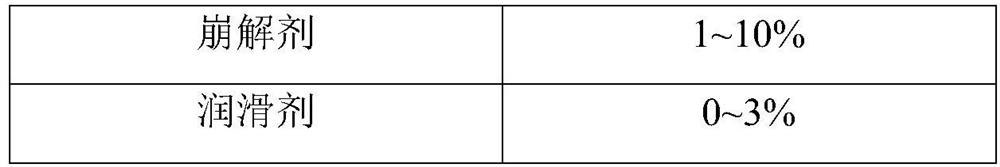
[0138] Nanocrystalline composition: take the obtained nanocrystalline solidified produc...
Embodiment 2
[0141] Preparation of nanocrystal suspension: dissolve stabilizer hypromellose E5 and Tween 80 in water to prepare a stabilizer aqueous solution, add lurasidone hydrochloride, and stir well; the obtained lurasidone hydrochloride is suspended The liquid was added to the ball mill, and 0.3 mm zirconia beads were added, the peristaltic pump was turned on at 22 rpm, the grinding chamber speed was 10 m / s, and the lurasidone hydrochloride nanocrystal suspension was obtained by wet grinding for 4 h.
[0142]
[0143] Nanocrystalline solids were prepared in a manner similar to that described in Example 1.
[0144]
[0145] Nanocrystalline compositions were prepared in a manner similar to that described in Example 1 .
[0146]
[0147]
Embodiment 3
[0149] Nanocrystal suspensions were prepared in a similar manner as described in Example 2.
[0150]
[0151] Nanocrystalline solids were prepared in a manner similar to that described in Example 1.
[0152]
[0153] Nanocrystalline compositions were prepared in a manner similar to that described in Example 1 .
[0154]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com