Selenium-containing teriparatide and fusion polypeptide for promoting osteoblast differentiation and application of selenium-containing teriparatide and fusion polypeptide
A technology of osteoblast differentiation and teriparatide, which is applied in the field of active peptides, can solve the problems that have not yet been determined, the nutritional and physiological roles of selenium and bone health are not clear, and growth retardation, so as to promote the mineralization of osteoblasts and alleviate the Pain, the effect of treating osteoporosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
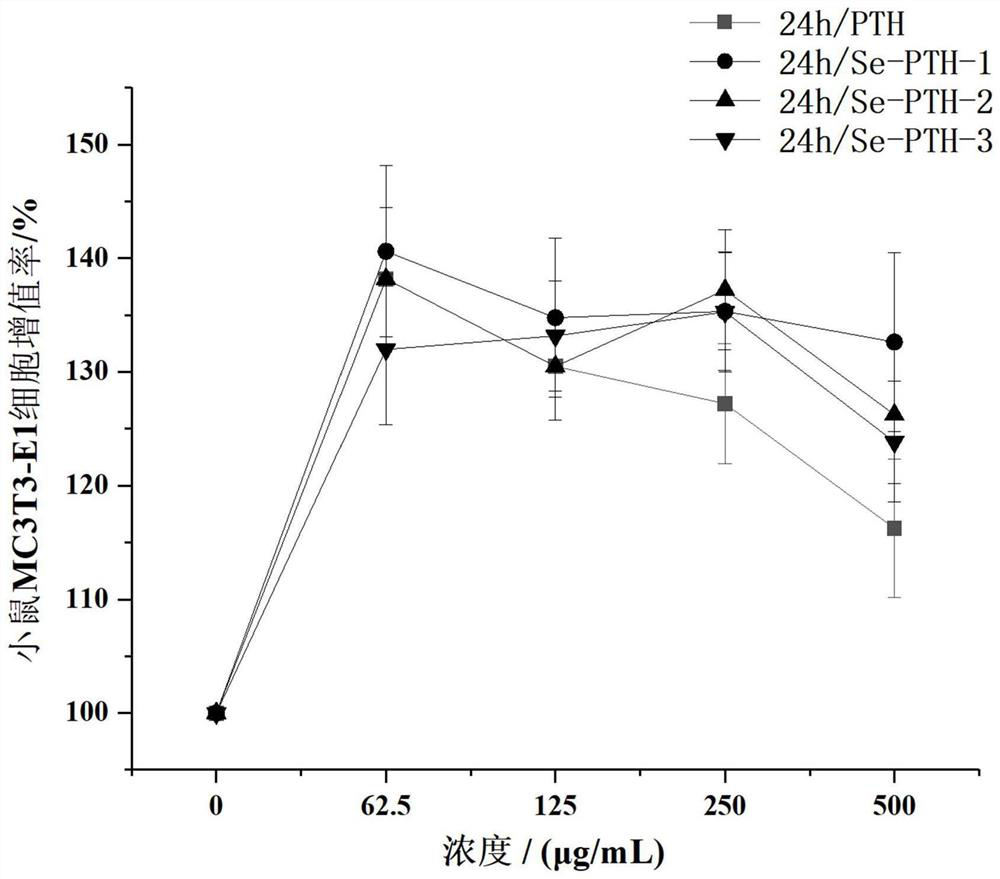
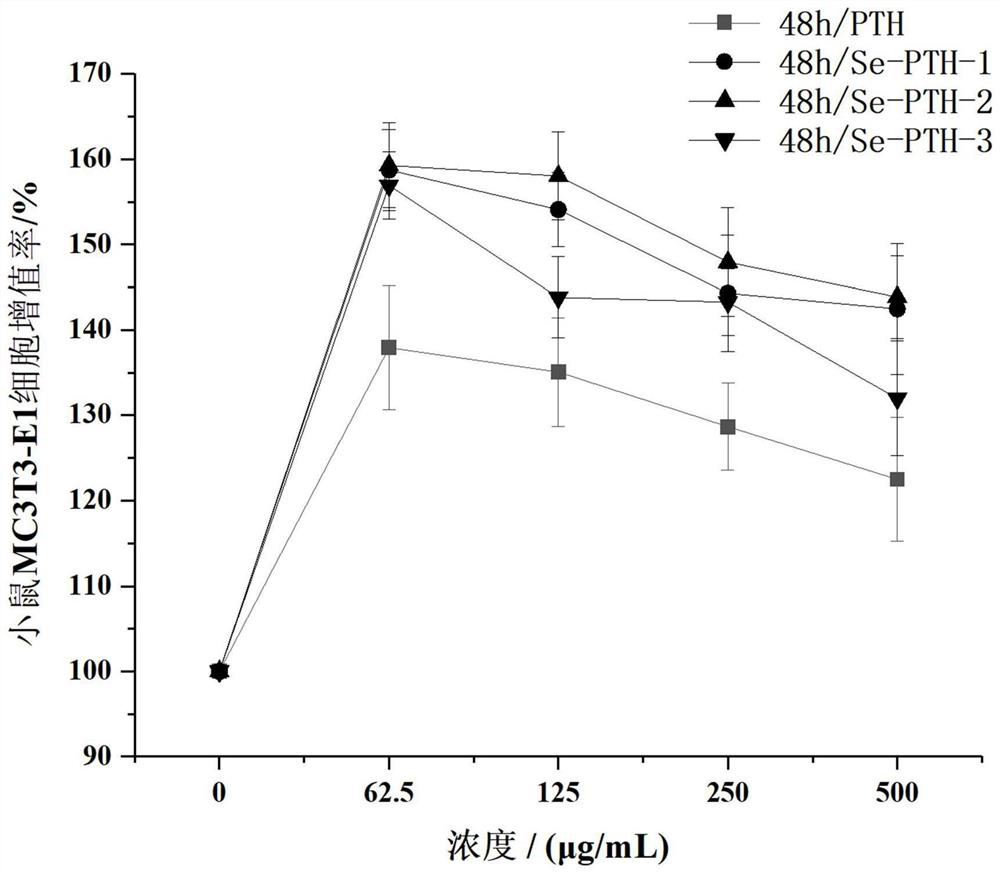
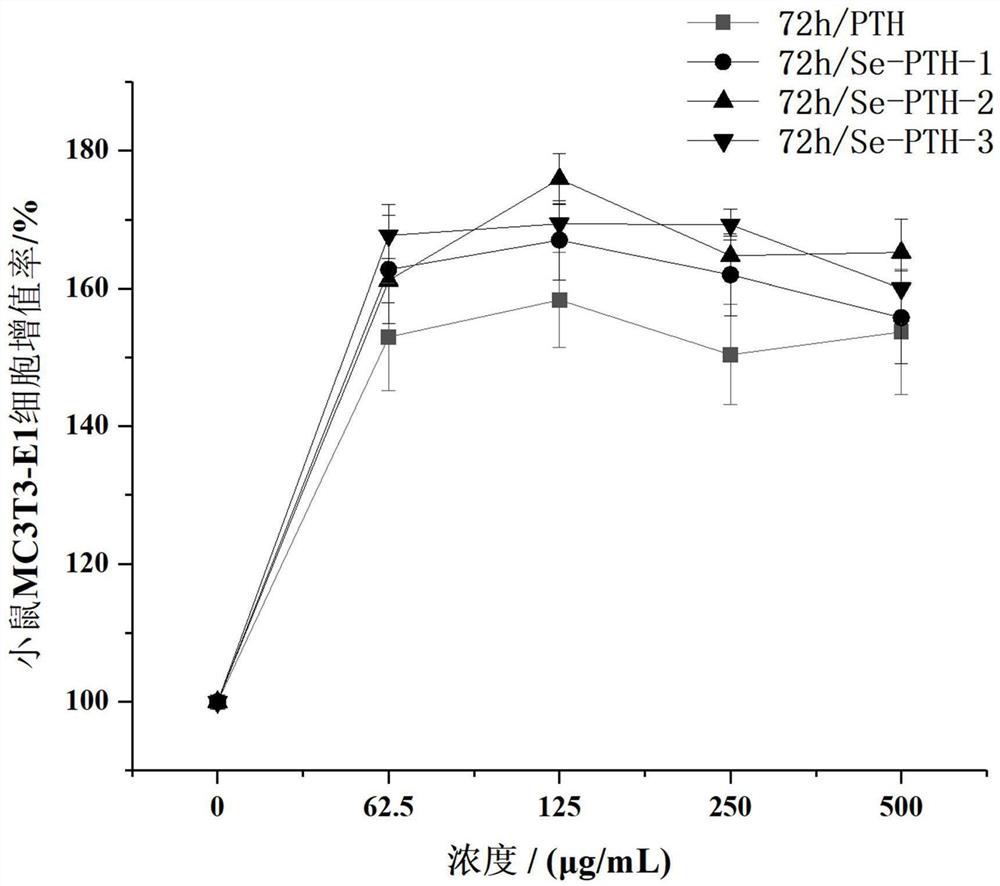
[0031] Example 1 The effect of selenium-containing teriparatide on the proliferation of mouse osteoblasts MC3T3-E1
[0032] based on Figure 1-Figure 3 The amino acid sequences of three kinds of teriparatide containing selenium were designed and obtained, and experimental samples were obtained by solid-phase synthesis method to verify the proliferation activity of osteoblasts. Solid-phase synthetic peptide company: Gill Biochemical (Shanghai) Co., Ltd.
[0033] Detection of proliferation rate of mouse osteoblast MC3T3-E1:
[0034] Select osteoblasts MT3T3-E1 in log phase growth, according to 2 × 10 4 Cells were added to a 96-well plate at a density of cells / mL, with 100 μL of cell suspension per well. The experiment was set to 5 groups. Blank control group: only DMEM medium containing 1% fetal bovine serum was added; positive control group: except for the addition of 1% fetal bovine serum. In addition to the DMEM medium containing 1% fetal bovine serum, teriparatide was add...
Embodiment 2
[0035] Example 2 The effect of selenium-containing teriparatide on alkaline phosphatase in mouse MT3T3-E1 cells
[0036] Detection of MT3T3-E1 alkaline phosphatase activity in mouse osteoblasts:
[0037] Select osteoblasts MT3T3-E1 in log phase growth, according to 2 × 10 4 Cells were added to a 96-well plate at a density of cells / mL, with 100 μL of cell suspension per well. The experiment was set to 5 groups. Blank control group: only DMEM medium containing 1% fetal bovine serum was added; positive control group: except for the addition of 1% fetal bovine serum. In addition to the DMEM medium containing 1% fetal bovine serum, teriparatide was added with final concentrations of 0; 62.5; 125; 250; 500ug / mL; No. 1 peptide group: except for the addition of DMEM containing 1% fetal bovine serum. In addition to the base, the final concentrations of 0; 62.5; 125; 250; 500ug / mL were added with No. 1 selenium-containing teriparatide; No. 2 peptide group: In addition to the addition o...
Embodiment 3
[0038] Example 3 The effect of selenium-containing teriparatide on mineralized nodules of mouse MT3T3-E1 cells
[0039] Determination of mineralized nodules in mouse osteoblasts MT3T3-E1:
[0040] Select osteoblasts MT3T3-E1 in log phase growth, according to 1 × 10 5 Add cells / mL to a 6-well plate, 2 mL of cell suspension per well, aspirate and discard the original medium after culturing for 24 hours, and replace it with a medium without fetal bovine serum. Culture medium without fetal bovine serum, the experiment was set to 5 groups, blank control group: only DMEM medium containing 1% fetal bovine serum and three osteogenic inducers (dexamethasone 10nmol, β-glycerophosphate sodium 10mmol) were added. , ascorbic acid 5 μg / ml); positive control group: except for the addition of DMEM medium containing 1% fetal bovine serum and three osteogenic inducers (dexamethasone 10 nmol, β-glycerophosphate 10 mmol, ascorbic acid 5 μg / ml), Teriparatide with a final concentration of 250ug / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com