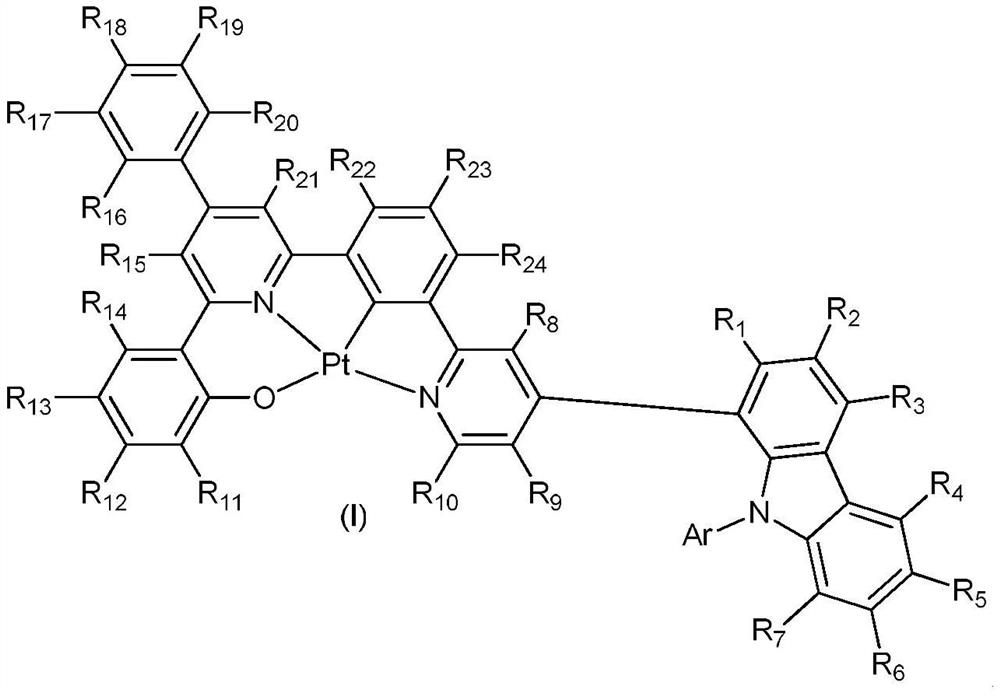
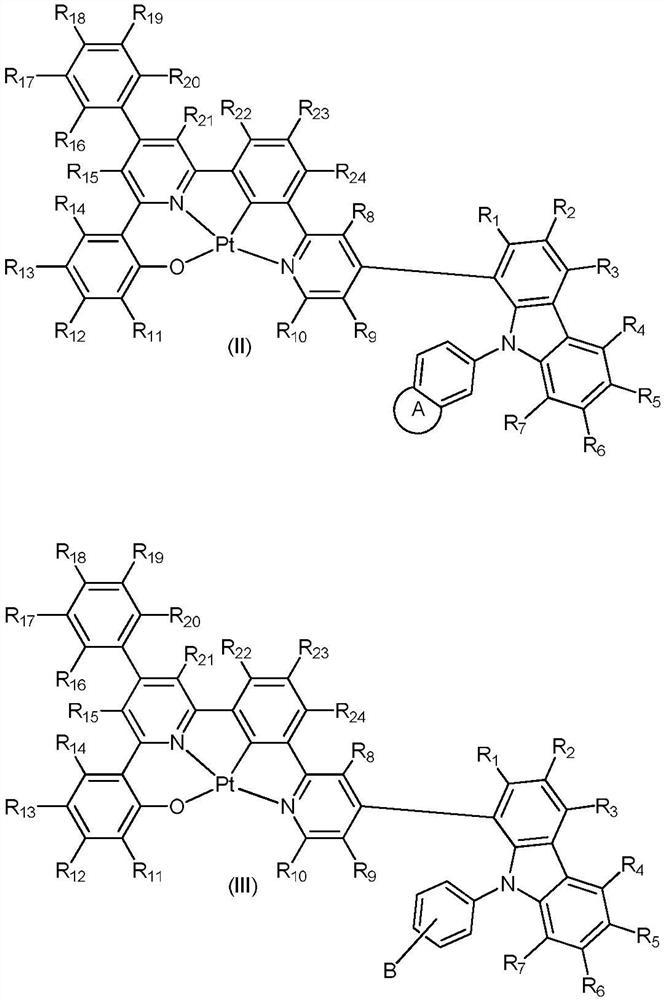
Platinum complex of ONCN tetradentate ligand containing carbazole
A technology of platinum complexes and tetradentate ligands, which is applied in the field of OLED materials to achieve high-efficiency conversion, simple synthesis steps, and reduced aggregation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
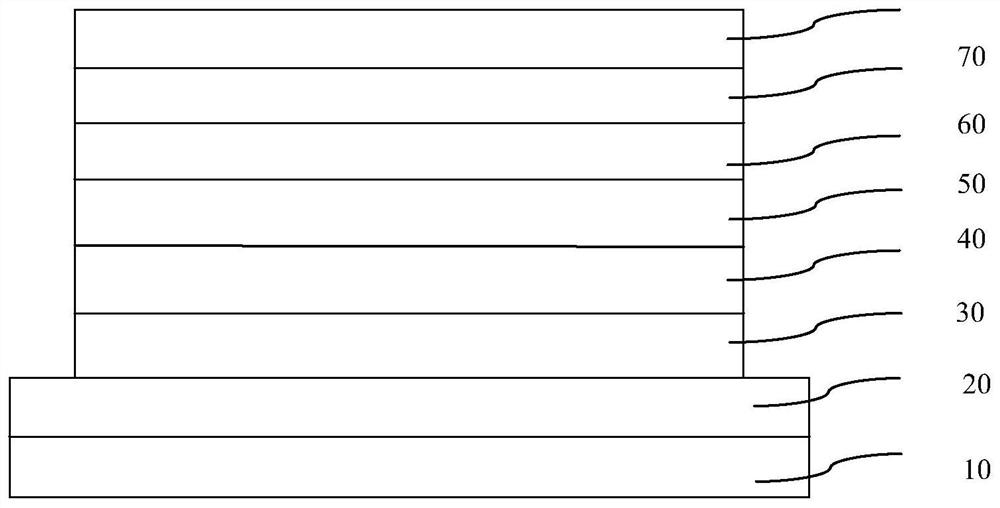
Image
Examples
Embodiment 1
[0035] Example 1: Synthesis of Intermediate G
[0036]
[0037] Synthesis of Intermediate C
[0038] Take a 1000ml three-necked bottle and put in A (10.0g, 40mmol, 1.0eq.), B (11.7g, 50mmol, 1.25eq.), Pd 132 (280mg, 0.4mmol, 1%eq.), K 2 CO 3 (13.8g, 100mmol, 2.5eq.) and toluene / ethanol / H 2 O (200 / 200 / 50ml), under nitrogen protection, the reaction was stirred at 90°C for 12h. After the reaction, most of the reaction solution was spin-dried, deionized water was added, extracted with dichloromethane three times, and the mixture was spin-dried and mixed with silica gel to pass through the column (Hex:EA=10:1). Finally, 21.0 g of a brown solid was obtained (yield 94.2%). The hydrogen spectrum data are as follows:
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.52(s, 1H), 8.31(d, J=5.2Hz, 1H), 8.16–8.07(m, 2H), 7.46(dd, J=9.1, 2.6Hz, 3H), 7.31(s, 1H) ), 7.28(s, 1H), 7.20(dd, J=5.3, 1.4Hz, 1H), 7.08(s, 1H), 4.03(s, 3H).
[0040] Synthesis of Intermediate D
[0041] Take 250ml sing...
Embodiment 2
[0048] Example 2: Synthesis of Complex 2
[0049]
[0050] Synthesis of Intermediate 2b
[0051] In a 250ml three-necked flask, add G (5.0g, 1.0eq), 2-iodonaphthalene (5.50g, 3.0eq), Cu (230mg, 0.5eq), CuI (688mg, 0.5eq), 1,10-Philo Linen (1.30g, 1.0eq) and cesium carbonate (7.06g, 3.0eq), 100ml of anhydrous xylene as the reaction solvent, nitrogen protection, oil bath temperature 160 ℃ for 3d reaction, cooled to room temperature, the reaction solution was rinsed with EA The agent was directly filtered to remove inorganic salts, and then stirred with silica gel column chromatography (chromatographic liquid hex:EA=8:1), and 3.8 g of yellow fluorescent product spots were collected. The hydrogen spectrum data is as follows 1 H NMR (400MHz, CDCl 3 )δ8.30(dd,J=5.9,3.1Hz,1H),8.24(d,J=8.2Hz,2H),8.18(s,2H),8.07(d,J=4.1Hz,2H),7.89( s, 1H), 7.59(t, J=5.1Hz, 6H), 7.48(d, J=8.6Hz, 2H), 7.45–7.30(m, 8H), 7.29(s, 1H), 7.17(s, 2H ), 7.06(dd, J=8.7, 5.1Hz, 3H), 3.91(s, 3H), 1.43(s, 18...
Embodiment 3
[0055] Example 3: Synthesis of complex 19
[0056] Synthesis of complex 19
[0057]
[0058] Synthesis of Intermediate 19b
[0059] Get 250ml there-necked flask, drop into intermediate G ((2.5g, 3.61mmol), 20a (2.02g, 7.22mmol), Cu (115mg, 1.81mmol), CuI (345mg, 1.81mmol), CsCO 3 (3.53 g, 10.83 mmol), o-phenanthroline (651 mg, 3.61 mmol) and xylene (50 ml), under nitrogen protection, the reaction was stirred at 160° C. for 30 h. After the reaction, it was directly filtered with suction, rinsed with EA, and spin-dried to obtain a brown solid, which was then passed through a silica gel column with (Hex:EA=5:1) to obtain 2.2 g of a white solid. The hydrogen spectrum data are as follows:
[0060] 1H NMR (400MHz, CDCl3) δ 8.49 (s, 1H), 8.42 (d, J=4.9Hz, 1H), 8.27 (dd, J=5.5, 3.5Hz, 1H), 8.19 (dd, J=14.2, 7.7Hz, 2H), 8.01 (d, J=6.4Hz, 2H), 7.90 (d, J=7.7Hz, 1H), 7.84 (d, J=1.1Hz, 1H), 7.57–7.47 (m, 4H) ,7.46(s,1H),7.38(ddd,J=22.4,10.0,5.3Hz,5H),7.28(d,J=8.1Hz,1H),7.22–7.07(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com