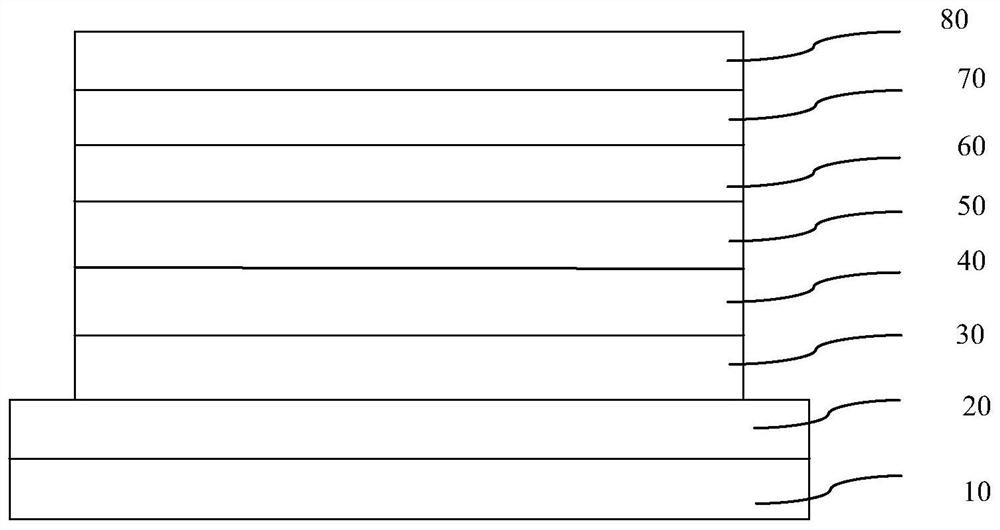
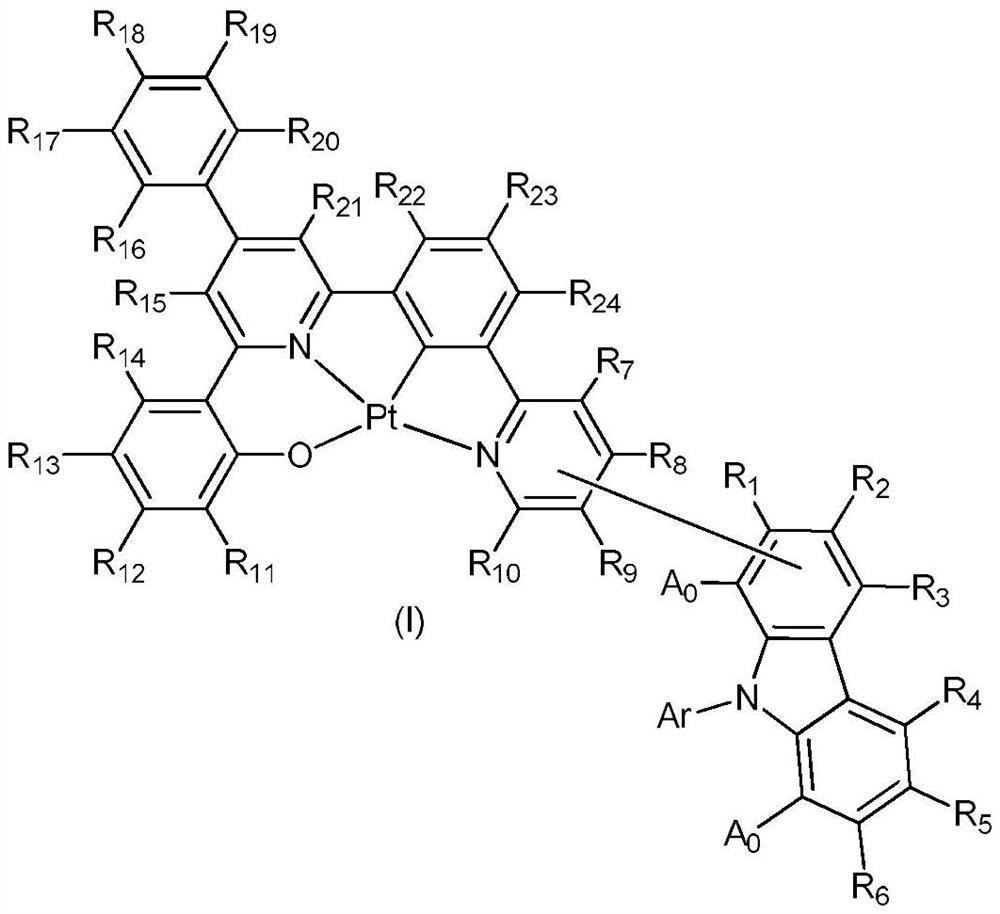
Divalent platinum complexes
A platinum complex, unsubstituted technology, used in the field of OLED materials, can solve the problems of high price of rare earth metal Ir, hinder application, and large pollution, and achieve the improvement of heavy atom effect, molecular spatial configuration, and low quenching constant. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of the compound of structure 1 (1a, refer to CN110872325A, 1d is the ordered material)
[0036]
[0037] Synthesis of Intermediate 1c
[0038] Take a 2L three-necked flask, put in 1a (60.0g, 101mmol), 1b (30.0g, 202mmol), Pd (PPh) 3 ) 4 (5.90g, 5mmol), NaOH (8.2mg, 202mmol) and dioxane / water (1.2L / 240ml), under nitrogen protection, the reaction was stirred at 55°C for 12h. After the reaction, most of the solvent was spin-dried, water was added, extracted twice with DCM, mixed with silica gel and spin-dried (Hex:EA=10:1) and passed through a silica gel column. 58 g of white solid were obtained. The hydrogen spectrum data are as follows:
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.69(s,1H),8.61(d,J=5.3Hz,1H),8.23(d,J=7.8Hz,1H),8.12–8.01(m,3H),7.91(d,J=1.4 Hz,1H),7.85(d,J=1.7Hz,1H),7.62(s,1H),7.55(s,3H),7.47-7.39(m,1H),7.30-7.26(m,1H),7.16 (s, 1H), 7.06(d, J=8.1Hz, 1H), 3.92(s, 3H), 1.41(s, 18H).
[0040] Synthesis of Intermediate 1e
[0041] Take a 250ml sin...
Embodiment 2
[0050] Example 2: Synthesis of the compound of structure 26 (26a is the ordered material)
[0051]
[0052] Synthesis of Intermediate 26b
[0053] Take a 250ml single-necked bottle, put in 1c (8.0g, 19.9mmol), 26a (8.5g, 21.4mmol), Pd 132 (0.303g, 0.42mmol), K 2 CO 3 (5.9g, 42.7mmol), and THF / water (80ml / 16ml), under nitrogen protection, react at 70°C for 12h. After the reaction, most of the solvent was spin-dried, water was added, extracted twice with EA, mixed with silica gel and spin-dried, and passed through a silica gel column with (Hex:EA=6:1) to obtain 11.08g of white solid. The hydrogen spectrum data are as follows:
[0054] 1 H NMR (400MHz, Chloroform-d) δ8.67 (d, J=4.5Hz, 1H), 8.22–8.17 (m, 3H), 8.17–8.08 (m, 3H), 7.95–7.86 (m, 4H), 7.72(dd,J=6.8,2.4Hz,1H),7.69-7.59(m,2H),7.53-7.27(m,10H),7.15(ddd,J=8.7,7.5,1.2Hz,1H),6.90( dd,J=7.7,1.2Hz,1H),3.90(s,3H),1.35(s,36H).
[0055] Synthesis of Intermediate 26c
[0056] Take a 500ml single-neck bottle, put in 26b...
Embodiment 3
[0060] Example 3: Synthesis of the compound of structure 36 (36a is the ordered material)
[0061]
[0062] Synthesis of Intermediate 36c
[0063] 36a in a dry 500ml double-necked flask (14.7g, 50mmol), add 36b (11.8g, 50mmol), toluene (120mL), add ethanol (60mL) and 2mol / L potassium carbonate solution (60mL), first ultrasonic 5- After 10 minutes, the nitrogen gas was rapidly stirred for 5 minutes, the catalyst tetrakis(triphenylphosphine) palladium (1.8 g, 1.5 mmol) was rapidly added, and a large amount of nitrogen gas was passed for 10 minutes. It was heated to 100° C., stirred for 12 hours, extracted first during treatment, spin-dried, and chromatographed with petroleum ether and dichloromethane to obtain 14.5 g of a white solid product with a yield of 90%. The hydrogen spectrum data are as follows:
[0064] 1 H NMR (400MHz, Chloroform-d) δ 9.54 (s, 1H), 8.37 (dd, J=4.0, 2.2Hz, 1H), 8.17–8.10 (m, 2H), 7.94 (dd, J=7.5, 2.2 Hz,1H),7.56(s,1H),7.55–7.48(m,2H),7.46(dd,J=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com