Ophthalmic drug emulsion composition as well as preparation method and application thereof
A composition and emulsion technology, which is applied in drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of temperature sensitivity, poor reducibility, pain, etc., and achieve the effect of less stimulation, stable product performance, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
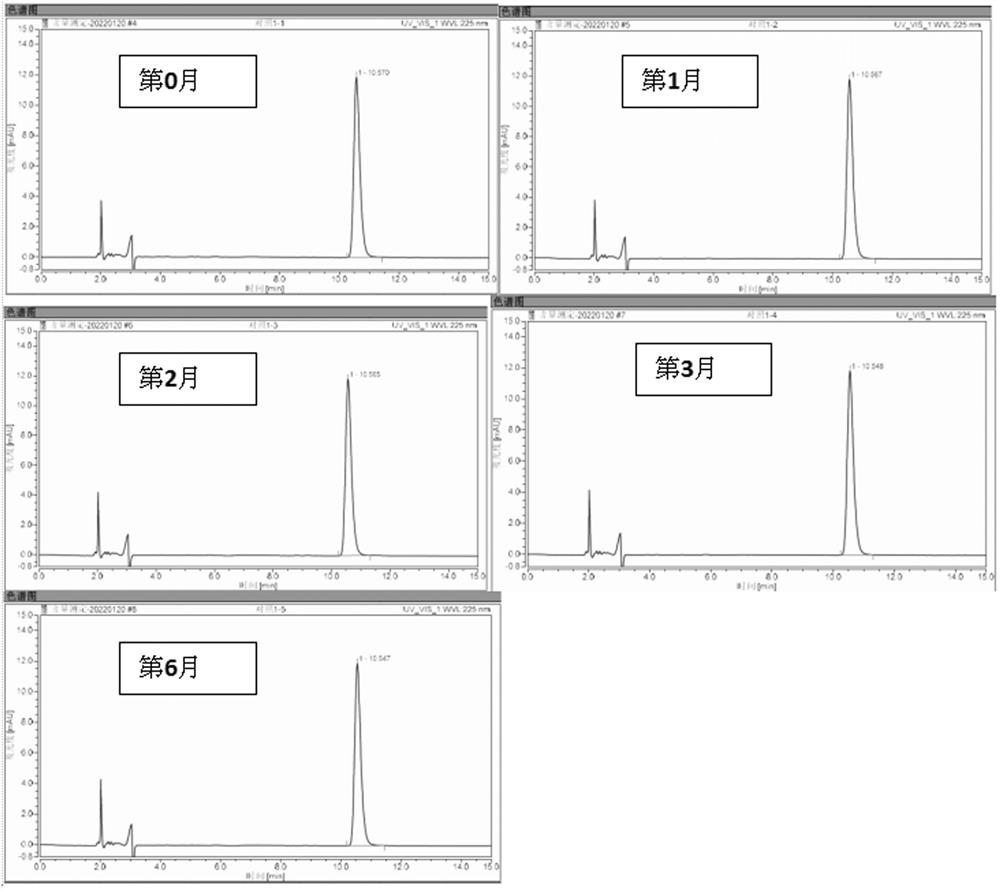
Examples
Embodiment 1
[0045] The compositions were prepared according to the components shown in Table 1, and three compositions of group 1, group 2 and group 3 were obtained respectively.
[0046] Table 1
[0047]
[0048] Preparation:
[0049] 1) Dissolve γ-cyclodextrin in water, add atropine sulfate, place it in an ultrasonic vibration cleaning machine, and ultrasonically mix for 30 minutes;
[0050] 2) Dissolve CKC, osmotic pressure regulator glycerol and tromethamine in the water phase;
[0051]3) Dissolve Polysorbate 80, Octoxynol 9, Tyloxapol or Poloxamer 188 in the oil phase;
[0052] 4) Heat the water phase and the oil phase to an appropriate temperature of 70-85°C, slowly pour the oil phase into the water phase under rapid mechanical stirring, continue stirring for 10 minutes, add water for injection to the full amount, and mix by high shear homogenization at least After 30 minutes, the oil droplets were reduced as much as possible, and the pH value was adjusted to 6.0 with dilute h...
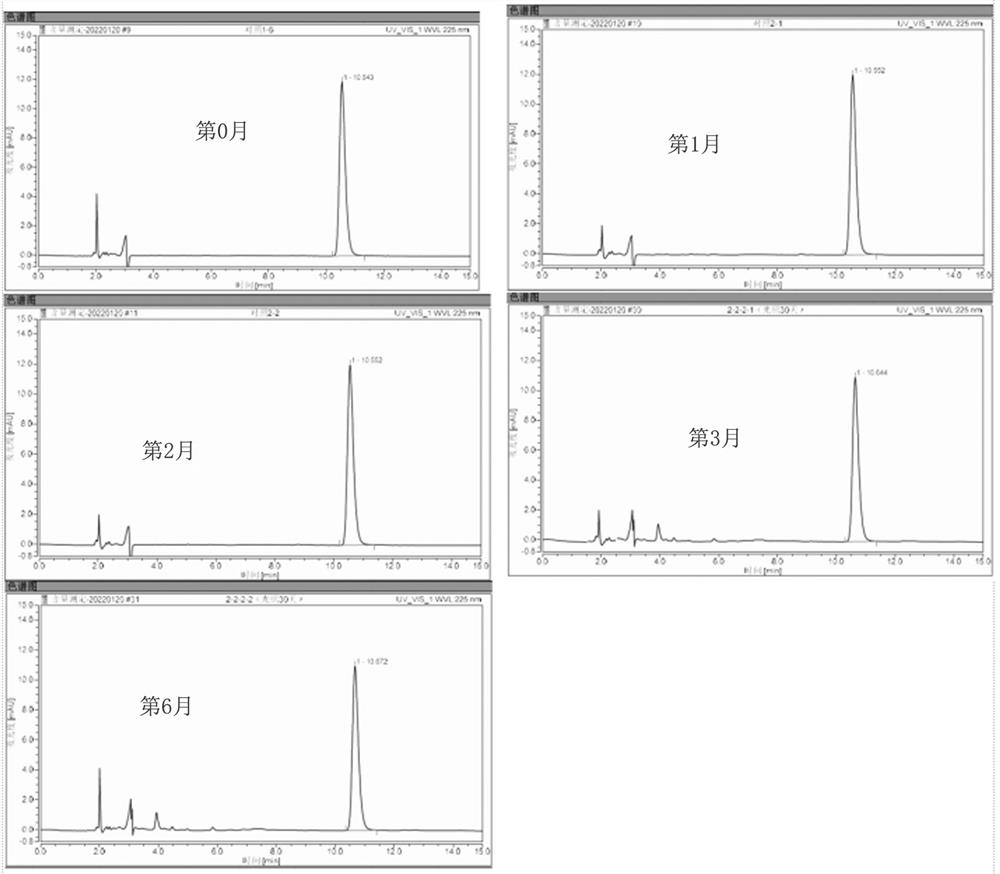
Embodiment 2
[0054] The compositions were prepared according to the components shown in Table 2, and three compositions of group 4, group 5 and group 6 were obtained respectively.
[0055] Table 2
[0056]
[0057] Preparation:
[0058] 1) Weigh β-cyclodextrin and dissolve it in water, then add atropine, place it in an ultrasonic vibration cleaning machine, and ultrasonically mix for 30 minutes;
[0059] 2) Dissolve CKC, osmotic pressure regulator glycerol, boric acid and sodium borate in the water phase;
[0060] 3) Dissolve Polysorbate 80, Octoxynol 9, Tyloxapol or Poloxamer 188 in the oil phase;
[0061] 4) Heat the water phase and the oil phase to an appropriate temperature of 70-85°C, slowly pour the oil phase into the water phase under rapid mechanical stirring, continue stirring for 10 minutes, add water for injection to the full amount, and mix by high shear homogenization at least After 30 minutes, the oil droplets were reduced as much as possible, and the pH value was adjus...
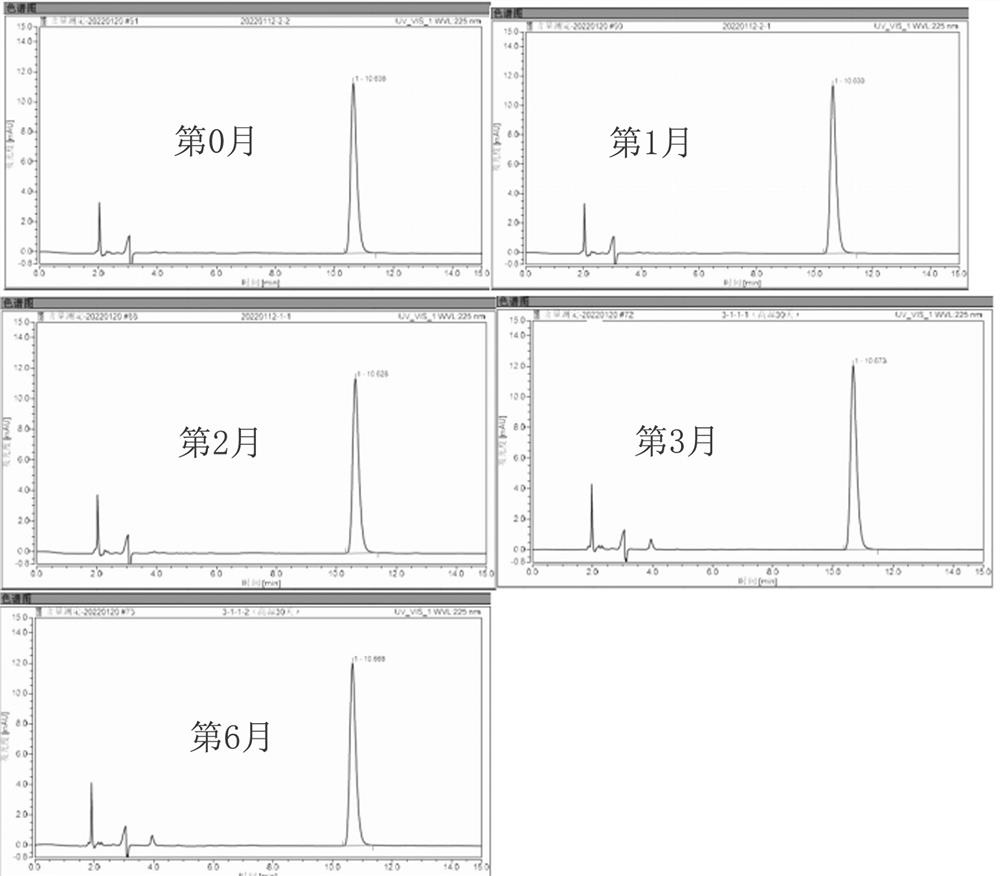
Embodiment 3
[0063] The compositions were prepared according to the components shown in Table 3, and three compositions of group 7, group 8 and group 9 were obtained respectively.
[0064] table 3
[0065]
[0066] Preparation:
[0067] 1) Weigh α-cyclodextrin and dissolve it in water, then add atropine, put it in an ultrasonic vibration cleaning machine, and ultrasonically mix for 30 minutes;
[0068] 2) Dissolve CKC, osmotic pressure regulator glycerol, boric acid and sodium borate in the water phase;
[0069] 3) Dissolve Polysorbate 80, Octoxynol 9, Tyloxapol or Poloxamer 188 in the oil phase;
[0070] 4) Heat the water phase and the oil phase to an appropriate temperature of 70-85°C, slowly pour the oil phase into the water phase under rapid mechanical stirring, continue stirring for 10 minutes, add water for injection to the full amount, and mix by high shear homogenization at least For 30 minutes, the oil droplets were minimized, and the pH was adjusted to 4.0 with dilute hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com